eISSN: 2575-906X


Research Article Volume 1 Issue 3
Department of Microbiology and Microbial Biotechnology, Saints Cyril and Methodius University of Skopje, Macedonia
Correspondence: Natalija Atanasova-Pancevska, Department of Microbiology and Microbial Biotechnology, Institute of Biology, Faculty of Natural Sciences and Mathematics, Saints Cyril and Methodius University of Skopje, Arhimedova 3, Skopje, Macedonia
Received: August 18, 2017 | Published: October 20, 2017
Citation: Atanasova-Pancevska N, Kungulovski D. Biodiversity of anaerobic fungi from ruminants in republic of Macedonia. Biodiversity Int J. 2017;1(3):100-107. DOI: 10.15406/bij.2017.01.00016
Anaerobic chytridiomycete fungi are found in the gastrointestinal tracts of sheep, cattle and goats, as well as in many other domesticated ruminant and nonruminant herbivores and a wide variety of wild herbivorous mammals. They are principally found associated with the fibrous plant particles of digesta and as free swimming zoospores in the fluid phase. The presence of large fungal populations in animals consuming mature pasture or diets largely composed of hay or straw together with the production of highly active fibre degrading enzymes lead to the belief that anaerobic fungi may have a significant role to play in the assimilation of fibrous feeds by ruminants. This paper is focused on anaerobic fungi because of their unusual biology and metabolism, and the biotechnological potential of their cellulases.
Keywords: Anaerobic fungi, Chytridiomycete, Ruminant, Herbivores, Zoospores, Cellulases
Herbivores as well as other vertebrates are not capable of producing cellulases and/or hemicelullases. Instead, a lot of herbivores form symbiotic associations with bacteria, protozoa and fungi which produce these enszymes and therefore are capable of degrading the plant polymers. In return for the relatively constant environment and continuous flow of plant material, the microorganisms of the herbivores’ digestive system provide the animal with easily usable form of carbon and energy, and ruminants with microbial proteins. Microbial eco-system of rumen is stable and at the same time dynamic.1 Stability is seen in the fact that the system itself is set well, in function and bioconversion of food in volatile fatty acids. In healthy ruminants, there is no contamination of eco-system although millions of microorganisms attack the rumen every day via food, water and air. Namely, this happens because rumen-microorganisms are adapted to survive the conditions in the rumen, whereas contaminants do not survive. They are anaerobyosis, high buffer capacity and osmotic pressure. On the other hand, the eco-system is dynamic due to the change of microbial population by changing food and frequency of feeding.
The only ultra structure of anaerobic fungi, their adaptation of gastrointestinal tract of herbivores and their habitation among phylogenetically different animals teaches us that they could exist as a separate group until the time when these mammals had started diverging, at least 120 million years ago.2 Although comparatively a little is known for the taxonomy of anaerobic fungi, generally it is known that they are zoospore-producing fungi and should belong to the classis of Chytridiomycetes. The order Spizellomycetales in Chytridiomycetes was established by Barr3 with the division of Chytridiales in order to explain the differences in the ultra structure of zoospores. Anyway, there are a lot of similarities between the families and genera from both or dines (Spizellomycetales and Chytridiales). Six ordines are known: Neocallimastix, Piromyces, Orpinomyces, Anaeromyces, Caecomyces, and since recently Cyllamyces.4 In Republic of Macedonia this is the first study to process anaerobic fungi in domestic and wild rumen animals.
Source of organisms
Anaerobic fungi were isolated from rumen content and feces from more domestic and wild herbivorous mammals. Animals which took participation in this paper are given in Table 1. Feces was taken in several occasions around 10.00-10.30 am and was picked up fresh from the ground and was taken immediately to the laboratory. Also, feces which has been old only several days was used. Only in one case was feces used, directly taken from the moufflon’s large intestine. Rumen content was taken during slaughtering, direclty from rumen, after which was taken to laboratory immediately.
Animal |
Origin of the Animal (Type of Animal- Phylum) |
Cattle(Bos indicus) |
Z (R) |
Domestic yak (Bos gruniens) |
Z (R) |
Ankole-watusi (Bos vatusi) |
Z (R) |
Red deer (Cervus elaphus) |
Z, J (R) |
Fallow deer (Cervus dama) |
Z, J (R) |
Barbary sheep (Ammotragus lervia) |
Z (R) |
Pygmy goat (Capra nigra spp.) |
Z (R) |
Roe deer (Capreolus capreolus) |
Z, J (R) |
Mouflon (Ovis musimon) |
J (R) |
Wild water buffalo (Bos bubalus) |
D (R) |
Sheep (Ovis aries) |
D (R) |
Goat (Capra hircus) |
D (R) |
Chamois(Rupucapra rupicapra) |
J (R) |
Table 1 Animals used in this paper.
Z: ZOO; J: Protected Nature Reserve Jasen; D: Domestic Animal; R: Ruminant Animal
Medium for isolation and cultivation
Many of the cultivation techniques and media which are used in rumen-microbiology originate from Hungate5,6 who studied anaerobic rumen bacteria. These techniques were modified and upgraded in order to provide media and procedures which are nowadays used constantly in the anaerobic microbiology.7 With relatively few exceptions, these media and techniques are used today, together with anaerobic bags and procedures with Petri dish8,9 for isolation and studying of anaerobic fungi. The basic medium which was used to isolate anaerobic fungi, was the medium 10 (M10) of.10 Medium M10 is one of the numerous media used in anaerobic rumen-microbiology and for our region and climate, ideal. It contains rumen fluid and that is why it is described as complex or habitat-simulating; it is well buffered at pH from 6.5 to 6.8 with bicarbonate and/or phosphate buffers and might be solidified with 0.8-1.5% agar. The medium contains resazurin as redox indcator, micro and macrominerals, organic and/or inorganic nitrogen sources and chemically reducing agentives, sodium sulfate and/or L-cystin hydrochloride.11 Reducing agets and anaerobic techniques are essential during medium preparation. One or more antibacterial antibiotics, penicillin, streptomycin and chloramphenicol incorporate in the medium; this is essential during isolation of anaerobic fungi from rumen content and faecal samples.12 In fact, antibiotics are necessary because of the anaerobic fungi growth which is completely inhibited in co-culture in anaerobic rumen bacteria.
In order to isolate axenic cultures from anaerobic fungi from their natural environment, free from contaminated bacteria, it is essential that repetitive subcultures are used, antibacterial antibiotics and some form of physical separation, such as cultivation of isolated colonies of agar medium.
Determination of morphological features of obtainedisolates
In order to study the morphology and anatomy of the obtained pure isolates, cultivated in liquid medium M10, they have been additionally observed by applying the lighting and fluorescent microscopy, in different stages of their development. For that puprose, there had been a native preparation which was coloured with safranin and was directly observed. To observe some of the isolates, for which the lighting microscopy did not give enough data, fluorescent microscopy was also used where a drop of suspension was mixed with fluorochrome colour and bisbenzimide (5mg/l PBS) to color the nuclei. The Nicleiturn fluorescent when lightened by UV light. They were determined accordingly the morphology of colonies, volume of fungal rhizoids and appearance of zoospores, with regards to.13
There are two morphological forms, monocentric(one reproductive body) and polycentric(more centres of reproduction). These forms are determined in the earliest stages of development and are invariable. In both forms, after zoospore encystation, the cyst germinates with producing germinative tube. In monocentric types, the nucleus does not enter the germinative tube. The germinative tube develops in rhizodal system with determined length. Anuclear rhizoids have double usage, grounding and absorption of nutrients. Rhizoids are of two types: typical filamentous form in Neocallimastix and Piromyces, and bulbous form in Caecomyces.
In polycentric types (Orpinomyces and Anaeromyces) the nucleus migrates outside the zoospore cyst in the germinative tube. The zoospore cyst does not have a further function in the development, but the cyst wall might remain. The germinative tube elongates and ramifies in mycelium (rhizomycelium) similar to other filamentous fungi. The nucleus ramifies constantly and migrates along the individual hyphae. This form of development results in mycelium thallus with unpredictable volume with many sporangia. Unfortunately, after the continuous culture, many of the mycelium polycentric types either produce sporangia which do not differentiate in zoospores, or they do not produce sporangia anymore, and their identification becomes problematic.
In identifying chytrids, it is essential not to forget their natural morphological variation. This variation complicates the identification and classification of these organisms. Because of the temperature needs and anaerobic status of anaerobic fungi, the problem is amplified and compared to the other chytrids, growth and development of one thallus cannot be studied microscopically under normal circumstances. The differences in the medium probably contribute to more variations rather than any other factor. When the medium is too rich, as in the glucose or in filter paper, the sporangia often become abnormally big and abort. Small but mature thalli of monocentric types, except C.communis, are similar and only by observing of the type of discharge of zoospores and zoospores flagellation, they might differ. When anaerobic fungi are identified and classified, it is essential to make sure the sporangia are healthy and viable, and conclusions should be reached only after enough material had been observed.
In addition, there is a description of obligatory anaerobic fungi, which have been isolated during the preparation of this study. The description is based completely on the thallus morphology, as seen through the light microscope, in order to enable functional identification of genera and phyla.
Isolate EZ1
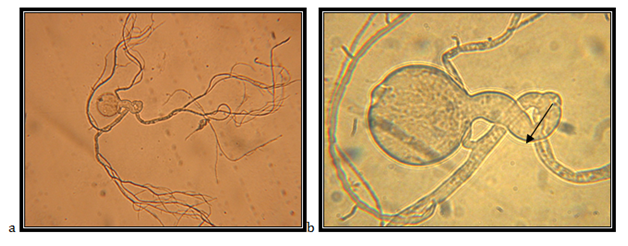
Endogenously developed zoosporangia, spheric with diameter of 65.71mm; rhizoids mainly come over from one axis, occasionally two or three at the same side of the sporangium, the neck is wide; the main axis close to the sporangium, up to 20 mm in diameter; main rhizoid is often coiled; rhizoids might have strongly narrowed places, rhizoid system is spread up to 1 mm in diameter, zoospores are liberated through apical pore.
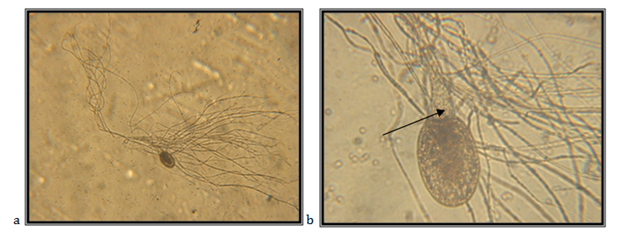
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the ZOO, Skopje. Accordingly the key to determine the anaerobic fungi of,13 the description of the isolate corresponds completely to the description of Neocallimastix frontalis.
In Malaysia, N. frontalis has been isolated from rumen material and droppings from a buffalo, ox, sheep, goat and deer.14 Also, rumen material has been isolated from oxen in New Zealand,15 Canada,16 Australia17 and USA 18 and from the rumen, the saliva and droppings from sheep in Great Britain19,20 (Figure 1 & 2).
Isolate EZ2
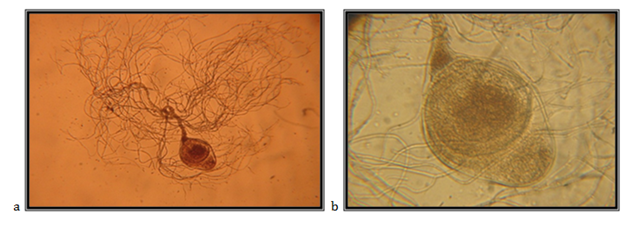
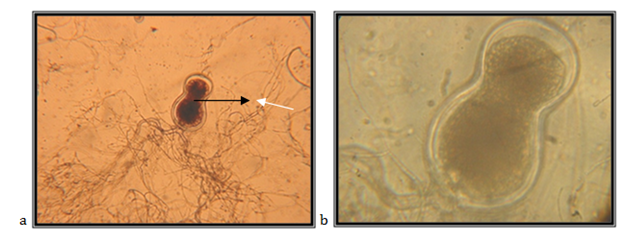
Endogenously developed zoosporangia, spherically elongated (egg-like); 157.14mm in diameter; rhizoids mainly come over from one axis, occasionally two or three at the same side of the sporangium, the neck is wide; the main axis close to the sporangium, up to 17.14mm in diameter, coiled; main rhizoid is often coiled; rhizoid might have strongly narrowed places; rhizoid system is spread up to 1 mm in diameter, zoospores are liberation through apical pore, accompanied by fast decomposition and cracking of the sporangium wall; zoospores are variable in length and shape, globular zoospores with 10-25mm in diameter, with 10 to 30 flagella, 35-50mm long.
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the ZOO, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastix frontalis (Figure 3).
Isolate EZ3
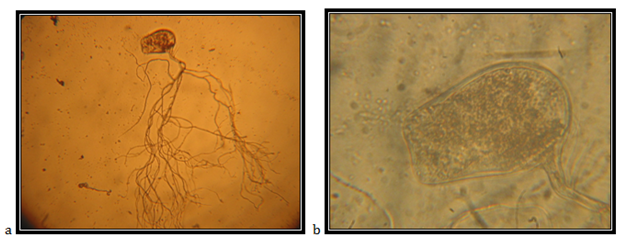
Zoosporangia, asymmetrically spheric, 161.24mm in diameter; widespread rhizoid, the neck is not narrowed or slightly narrowed, the main axis, close to the sporangium, up to 14.28mm in diameter, coiled; the main rhizoid is often coiled; rhizoid system is spread up to 600mm in diameter; zoospores are liberated through side pore, accompanied by fast decomposition and cracking of the sporangium wall; zoospores are variable in length and shape; spheric zoospores, polyflagellates.
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the ZOO, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastixspp (Figure 4 & 5).
Isolate EJ1
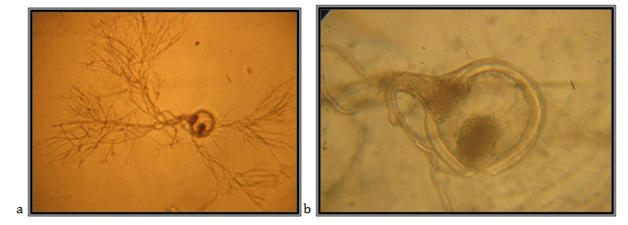
Zoosporangia, symmetrically spheric, 119.99mm in diameter; widespread rhizoid, the neck is slightly narrowed, the main axis, close to the sporangium, up to 25.71mm in diameter, coiled; the main rhizoid is often coiled; rhizoid system is spread up to 800mm in diameter; zoospores are liberated through side pore, accompanied by fast decomposition and cracking of the sporangium wall, which is clearly double-layered; zoospores are variable in length and shape; spheric zoospores, polyflagellates.
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastixspp (Figure 6).
Isolate SZ1
Monocentric sporangia, double pear-like 31*115mm; with several papillae; the main rhizoid is filamentously spread, it elongates up to 180mm,discharge follows after the decomposition of the papillae; zoospores are ovoid, 3.1-8.0mm, uniflagellate. The isolate is taken from feces of a roe deer (Capreolus capreolus), kept in the ZOO, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces mae.
In Malaysia, P. mae has been isolated from the rumen and feces of oxen, buffalos, sheep and goats, as well as from the duodenum of the sheep. Also, this type has been isolated from the rumen of Elk in Canada16 and in the rumen of sheep in France21 (Figure 7 & 8).
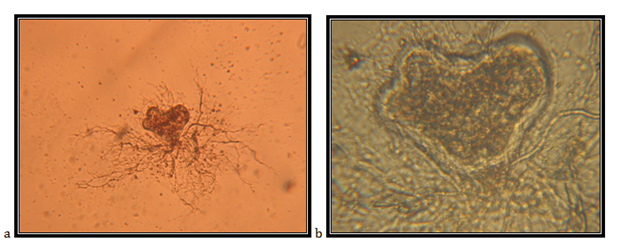
Isolate SJ1
Monocentric sporangia, heart-like, 48*89mm; with several papillae; the main rhizoid is filamentously branched, it elongates up to 330mm, discharge follows after the decomposition of one or two papillae; zoospores are spheric to ovoid, uniflagellate.The isolate is taken from feces of a roe deer (Capreolus capreolus), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces mae (Figure 9).
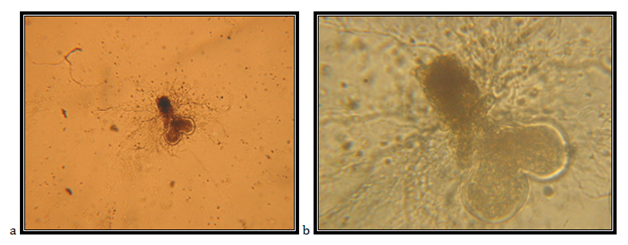
Isolate EJ2
Monocentric sporangia, endogenous, with diameter of 97mm; the main rhizoid is large, 583.13mm in diameter, without constrictions or slightly constricted in the neck area, branched with constrictions on the rhizoid; zoospore examination is with decomposition of a wide apical part of the wall; zoospores are quite variable in the shape, uniflagellate. The isolate is taken from feces of a fallow deer (Cervus dama), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces communis.
In Malaysia, P. communis has been isolated from the rumen of sheep, goat, ox, water buffalo and deer, and from the duodenum of the sheep.22 The coil of the main rhizoid is often found in the endogeneous forms. Piromyces communis is, also isolated from the rumen of sheep in Great Britain,23 and France,24 as well as from the rumen of oxen in Canada16 (Figure 10).
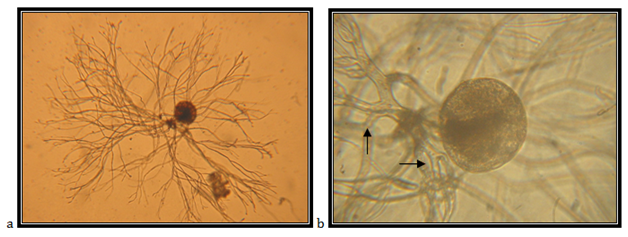
Isolate EJ3
Monocentric sporangia, endogeneous, ellipsoid with diameter of 31.42mm; rhizoids are sometimes with constrictions; the main rhizoid is usually not branched, ending in a poorly branched system; zoospores are uniflagellates. In cultures, this type is easy to differentiate from other Piromyces spp. because of how small the sporangium is. It has been found only in Malaysia, in the rumen of Javan rusa,25 goat, sheep and in the duodenum of sheep.22
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces minutus (Figure 11).
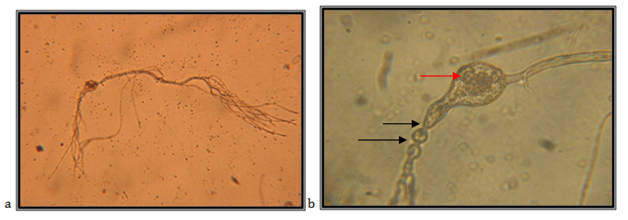
Isolate EJ4
Monocenttric; sporangia are elongated and ovale, 74.28*51.42mm; main rhizoid is filamentously branched , it elongates up to 800mm; sporangium might lay on both shorter and longer sporangiophore without constrictions; discharge of zoospore follows after the decomposition of the sporangium wall; zoospores are spheric to ovale, 2.0-11.0mm.
The isolate is taken from feces of a fallow deer (Cervus dama), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces mae (Figure 12).
Isolate SJ2
Monocenttric; sporangia are strictly endogeneous, ellipsoid or in a shape of pear, with diameter of 171mm; main rhizoid is not branched, ending in a branched system; zoospore discharge follows after a wide apical pore, occasionally two pores of a large sporangium; zoospores are uniflagellates.
In cultures, this type is easy to differentiate from other Piromyces spp. because of how small the sporangium is. It has been found only in Malaysia, in the rumen of Javan rusa,25 goat, sheep and in the duodenum of sheep.22 The isolate is taken from feces of a fallow deer (Cervus dama), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces minutus (Figure 13).
Isolate Z2
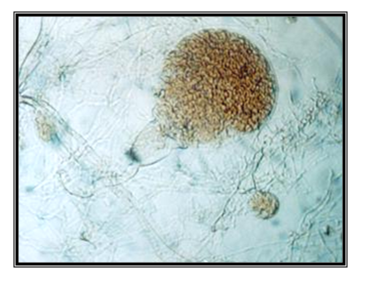
Endogenously developed zoosporangia, spheric, 83.61mm in diameter; rhizoids mainly come over from one axis, occasionally two or three at the same side of the sporangium, the neck is wide; the main axis close to the sporangium, up to 32mm in diameter, coiled; main rhizoid is often coiled; rhizoid system is spread up to 1 mm in diameter, zoospores are liberated through apical pore, accompanied by fast decomposition and cracking of the sporangium wall; zoospores are variable in length and shape, spheric zoospores 7-22mm in diameter, with 7 to around 30 flagella.
The isolate is taken from feces of a zebu (Bos indicus), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastix frontalis (Figure 14 & 15).
Isolate L2
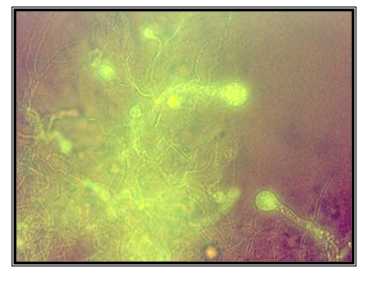
Mycelium with undetermined length is raised above the zoospore cyst in germination; polycentric. Zoospores were not found. Constrictions of the hyphas are in the shape of isthmus. Only because of the absence of zoospores, were we not able to determine for sure what genus of polycentric anaerobic fungi they were; but the presence of constrictions in shape of isthmus which are correctly positioned, means that these are polycentric fungi of genus Anaeromyces. The isolate is taken from the feces of llama (Lama glama), kept in ZOO, Skopje (Figure 16 & 17).
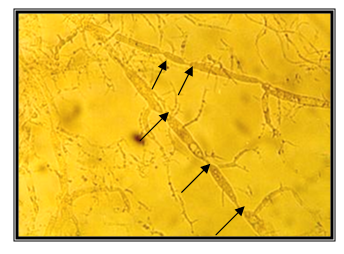
Isolate J2
Single longitudinal sporangia, 28*106mm, in the shape of bobbin (with apical bud), formed on sporangiophores, uniflagellates zoospores, and hyphas-constricted. The isolate is taken from feces of a domestic yak (Bos gruniens), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Anaeromyces elegans. This type can be often found in the rumen of oxen and buffalos in Malaysia (Figure 18 & 19).
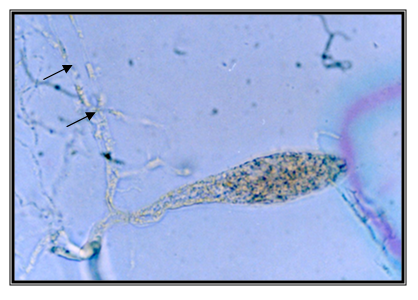
Isolate V4
Mycelium with undetermined length is raised above the zoospore cyst in germination; polycentric. Zoospores were not found. Constrictions of the hyphas. Only because of the absence of zoospores, were we not able to determine for sure what genus of polycentric anaerobic fungi they were.
The isolate is taken from the feces of watusi (Bos vatusi), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of polycentric genus of anaerobic fungi Orpinomyces spp. or Anaeromyces spp (Figure 20).
Isolate BO3
Monocentric; sporangia are double pear-like (heart-like), 39-61*89-128mm, with several papillae; main rhizoid is filamentously branched, elongated to 450mm; discharge follows after one or two papillae; spheric zoospores, 2.0-12.5mm, uniflagellate, rarely two to four flagella. The isolate is taken from the feces of a barbary sheep (Ammotragus lervia), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces mae (Figure 21).
Isolate ES1
Filamentous fungus, with monocentric thallus; with double oval sporangia which are kept on one sporangium with diameter of around 77mm. When they are mature, the upper half of the sporangium opens and zoospores are liberated. Zoospores have a diameter of 7.3mm; uniflagellate.
The isolate is taken from the feces of a red deer (Cervus elaphus), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces citronii (Figure 22).
Isolate J1
Monocentric; sporangia are elongated; main rhizoid is filamentously branched, elongated to 450mm; spheric zoospores, 2.0-12.3mm, uniflagellate, rarely two to four flagella. The isolate is taken from the feces of a yak (Bos gruniens), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Piromyces mae (Figure 23).
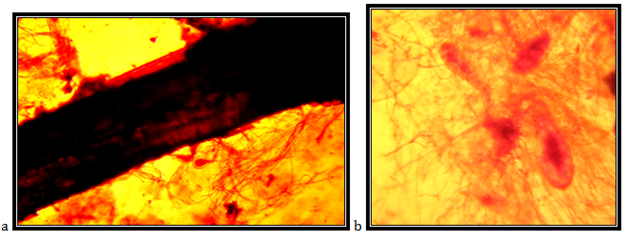
Isolate J3
Endogenously developed zoosporangia, ovally elongated; rhizoids mainly come over from one axis, occasionally two or three at the same side of the sporangium, the neck is wide; the main axis close to the sporangium, up to 18 mm in diameter, coiled; main rhizoid is often twirld; rhizoid system is spread up to 1 mm in diameter, zoospores are liberated through apical pore, accompanied by fast decomposition and cracking of the sporangium wall; zoospores are variable in length and shape, 5-18mm in diameter, with 7 to around 30 flagella.
The isolate is taken from the feces of a yak (Bos gruniens), kept in the ZOO in Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastix frontalis (Figure 24).
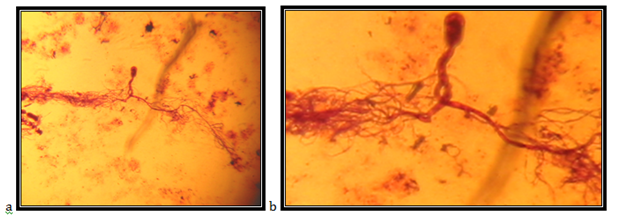
Isolate MR1
Endogenously developed zoosporangia, spheric with diameter of 42mm; rhizoids mainly come over from one axis, occasionally two or three at the same side of the sporangium, the neck is wide; the main axis close to the sporangium, up to 15mm in diameter, coiled; main rhizoid is often coiled; rhizoid system is spread up to 1 mm in diameter, zoospores are liberated through apical pore, accompanied by fast decomposition and cracking of the sporangium wall; zoospores are variable in length and shape, often spheric, with 7 to around 30 flagella.
The isolate is taken from the feces of a moufflon (Ovis musimon), kept in the Protected Nature Reserve Jasen, Skopje. Accordingly the key to determine the anaerobic fungi of13 the description of the isolate corresponds completely to the description of Neocallimastix frontalis (Figure 25).
The study reveals that 19 isolates of obligatory anaerobic fungi, which have been isolated during the preparation of this study. The description is based completely on the thallus morphology, as seen through the light microscope, in order to enable functional identification of genera and phyla. Among these, three isolates are identified as polycentric, and 16 are identified as monocentric. The present study gives a baseline data of biodiversity of anaerobic fungi from ruminants in Republic of Macedonia.
None.
The authors declare that there is no conflict of interests to declare regarding the publication of this paper.

©2017 Atanasova-Pancevska, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.