eISSN: 2575-906X


Storage conditions and containers are significant in preservation and maintenance of healthy seed during post harvest period. Different storage are employed to preserve the seed of pulses; commonly used containers are Gunny bag, Tin box, Plastic bag and Glass bottle. Study to investigate better storage container carried out by observing seed mycoflora, seed germination, shoot and root length of Chickpea seed. Among all containers employed to store the seed of pulse; Gunny bag found to be better storage container to maintaining seed health of test pulse.
Keywords: chickpea, annual plant, herbaceous, plants, polythene bags
Chickpea (Cicer arietinum L.) is herbaceous annual plant. The leaves are pinnately compound with papilionaceous flowers, pods contains one or two seeds. It is cultivated in dry cool climate during Rabbi Season in the regions of low to moderate rainfall. It is cultivated as intercrop along with Jowar, Wheat, Bajra etc during October-November. The crop is harvested after about 3-4 months in February – March. It is mainly cultivated in states Bihar, Maharashtra, Uttar Pradesh, Punjab, Rajasthan, Madhya Pradesh, Andhra Pradesh, West Bengal, Tamil Nadu and Karnataka, India.
Chickpea seed contain malic and oxalic acids from the leaves of Chick pea are used in intestinal disorders. Its protein content is 20.5g/100g of seeds and carbohydrates is 59.6g/100g of seeds along with thiamin (0.30mg), riboflavin (0.15mg), niacin (2.9mg), vitamin C (3mg) and phosphorous (312mg).1
Collection of test pulse, plants and preparation of plant parts powder
Chickpea (Cicer arietinum L.) collected from local farms and market in Nanded district of Maharashtra, India. The test treatment plants; Azadirachta indica A. Juss., Ocimum basilicum L. and Cyperus rotundus L.; used as bio-powders, collected from local area of Nanded district, Maharashtra, India and identified from their morphological characters using ‘Flora of Marathwada’.2 Plants were cut and separated into different parts stem, leaves, root and surface sterilized with 0.1% HgCl2 and washed to remove disinfectant; with sterile distilled water. Sterilized plant parts kept for drying in hot air oven at 60ºC for 48hours.
Application of plant part powders to seed of test pulse Chickpea
The dried plant parts leaf, stem and root crushed to powder with the help of grinder. The powders thus obtained passed through sieve to get fine powder and stored in polythene bags for the study.
One kilogram seed of Chickpea dusted separately with ten gram of leaf powder of Azadirachta indica A. Juss, Ocimum basilicum L. and rhizome powder of Cyperus rotundus L. Treated seed of the pulse were stored in different containers like gunny bag, plastic bag, tin box and glass bottle. After storing seed of test pulse in different containers for one year, the seeds were incubated on moist blotters for ten days at room temperature. On eleventh day seed health in terms of seed mycoflora, seed germination, root and shoot length was studied. Seeds without dusting with any plant part powder served as control.
The results in the Figure 1 show, seed from all the containers; treated with leaf powder of Azadirachta indica A. Juss. Showed reduced seed mycoflora and enhanced seed germination, shoot and root length. Seed mycoflora was considerably reduced in treated than in untreated seed stored in all storage containers. In untreated seeds maximum seed mycoflora was recorded in plastic bag (67%) and minimum in gunny bag (58%). In treated seeds maximum seed mycoflora was found in the seeds stored in plastic bag (36%), where as minimum in gunny bag (20%), followed by tin box (28%).
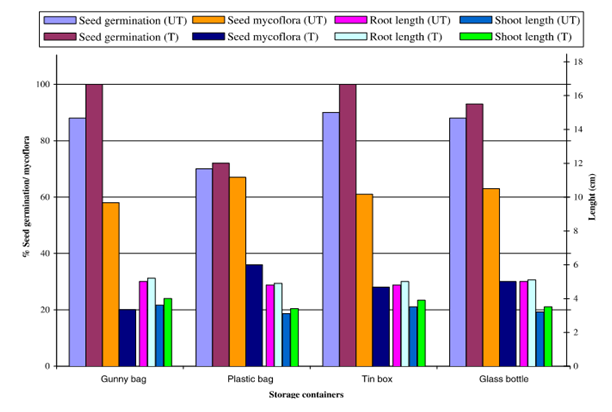
Figure 1 Effect of different storage containers on seed mycoflora and seed health (seed germination, shoot length and root length) of Chick pea (Cicer arietinum L.) seeds treated with leaf powder of Azadirachta indica A. Juss (UT, untreated; T, treated).
More seed germination was recorded in treated seeds compared to untreated one stored in all containers. Maximum seed germination in untreated seeds was found in the seed stored in tin box (90%), followed by gunny bag and glass bottle (88% each) and minimum in plastic bag (70%). Maximum seed germination in treated seeds was recorded in gunny bag and plastic bag (100% each) and least in plastic bag (72%). Increased shoot and root lengths were reported in treated seed than untreated one, stored in all containers. Root length in untreated and treated was slightly more in the seeds stored in gunny bag and plastic bag, similarly shoot length was a bit better in case of untreated and treated seeds stored in gunny bag and tin box.
Seed treated with rhizome powder of Cyperus rotundus L. indicated reduced seed mycoflora and increased seed germination, shoot and root length stored in all the containers. Reduced seed mycoflora was noticed in treated seeds than in untreated seeds stored in selected different storage containers (Figure 2). Untreated seeds stored in tin box recorded minimum seed mycoflora (59%), followed by gunny bag (60%). Maximum seed mycoflora in treated seeds was recorded in plastic bag (32%) and minimum in gunny bag (26%), followed by tin box (29%).

Figure 2 Effect of different storage containers on seed mycoflora and seed health (seed germination, shoot length and root length) of Chick pea (Cicer arietinum L.) seeds treated with rhizome powder of Cyperus rotundus L. (UT, untreated; T, treated).
Seed germination was found to be increased in treated seeds than in untreated seeds, stored in all containers. Untreated seeds stored in gunny bag showed maximum seed germination (88%) and minimum in plastic bag (70%). Treated seeds stored in tin box showed maximum seed germination (100%) followed by gunny bag (98%) and least was recorded in plastic bag (78%). Shoot and root lengths were increased in treated seeds compared to untreated stored in all containers. Treated seeds showed increased root and shoot lengths in all the containers.
Figure 3 show; Seeds treated with Ocimum basilicum L. showed reduced seed mycoflora and increased seed germination, shoot and root length in all the containers. Seed mycoflora of treated seeds was found to be reduced compared to untreated seeds stored in all containers. Untreated seeds stored in gunny bag showed minimum seed mycoflora (50%) and maximum was reported in seed from plastic bag (62%). Treated seeds stored in glass bottle showed maximum seed mycoflora (35%) where as minimum was in gunny bag seed (12%). Seed germination was more in treated seed compared to untreated seed, stored in all containers.
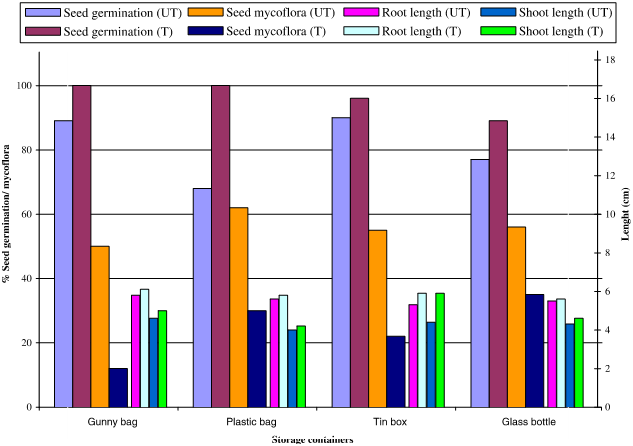
Figure 3 Effect of different storage containers on seed mycoflora and seed health (seed germination, shoot length and root length) of Chick pea (Cicer arietinum L.) seeds treated with leaf powder of Ocimum basilicum L. (UT, untreated; T, treated).
Maximum seed germination in case of untreated seeds was recorded in tin box seed (90%), followed by gunny bag (89%) and minimum in plastic bag (68%). Treated seeds stored in gunny bag and plastic bag showed maximum seed germination (100% each) where as minimum in seed stored in glass bottle (89%). Treated seeds, stored in all containers showed increased shoot and root lengths over untreated seeds. Untreated and treated seeds stored in gunny bag showed slightly more increased shoot and root lengths among all containers.
Similarly, Singh & Singh,3 found difference in fungal flora under different storage periods, four months stored seeds nurtured Chaetomium globosum, C. spirata, Rhizopus arrhizus and Penicillium sp. and eight month stored seeds developed mainly Aspergillus fumigatus, A. sydowii, A. flavus and A. niger. Chandra et al.4 studied seed mycoflora of mustard, linseed, sunflower, safflower, soybean, sesame and groundnut recorded that, the fungi like Alternaria, Cladosporium, Curvularia, Fusarium and Helminthosporium decreased gradually during storage period and disappeared after three years and were succeeded by storage fungi like Aspergillus sp. Penicillium spp. and Rhizopus sp. Bhattacharya et al.5 recorded fungal infection, moisture content, germinability and deterioration of seeds of maize, groundnut and soybean in storage at the locality of Santiniketan, West Bengal, India under natural condition for one year. Dominant fungi recorded from stored seeds were Aspergillus candidus, A. flavus, A. niger, A. terreus, A. ruber, Rhizopus sp. Penicillium sp., Curvularia sp., Fusarium sp. Alternaria sp. etc. Carbohydrates and protein content of the test seeds were found to be declined. Zeljko Jurjevic et al.6 studied changes in fungi and mycotoxins in pearl millet under controlled storage conditions; further they reported that, predominant fungi showed fluctuation in their incidence with changes in storage conditions such as temperature, moisture and humidity. Abdulaziz et al.7 reported that storage of Ephedra alta seeds in cotton cloth bags favorably maintained seed moisture content below critical level resulting in minimum seed deterioration compared with other seed storage containers. Khatun et al.8 used botanicals, such as whole leaf powder of neem (Azadirachta indica), Ipomoea sepiara, and Polygonum hydropiper at a dose of 5% w/w (25g botanical per 500g of lentil seeds), Azadirachta indica A. Juss. In addition, Polygonum hydropiper L. were effective in preserving seed germination and seed vigor of lentil. Mysore Rangnayaka et al.6 found that storage fungi reduced total fat (1.94-1.75g), triglycerides (1.46-1.07g), where as phospholipids (0.06-0.21g), free fatty acids (0.002–0.01g) and peroxide values increased. The fatty acid content of palmitic, steric, linoleic acid decreased, but oleic acid content increased in Red Gram and Chickpea during storage periods. Khalequzzman et al.9 found moisture content, seed weight, abnormal seedlings, seed rot, and fungal association of French bean increased, but germination and normal seedlings growth decreased with increase in storage period. Kakade & Chavan10 reported negative nutritional and fatty oil alteration in soybean and safflower due to storage fungi; like Alternaria, Fusarium, Macrophomina sp., Curvularia sp., Rhizopus Sp., Penicillium sp. etc. Sethumadhav Rao et al.11 reported that storage fungi like Aspergillus flavus, A. niger, A. fumigatus, Cladosporium cladosporiodes etc reduced carbohydrates, amino acids and phenols in the vegetables, increased storage period abnormally increased phenols and amount of reducing sugar. Dubale et al.12 screened seed mycoflora of Maize (Zea mays L.) stored in traditional storage container Gombisa and sacks, common fungi reported were Aspergillus flavus, A. fumigatus, A. niger, A. terreus, Cladosporium cladosporiodes, Drechslera halodes, Fusarium oxysporum and Penicillium chrysogenum. Lambat et al.13 reported polyethylene bag imparted much protection.
In vitro application of the selected plants could be used on larger scale in storage houses to eschew artificial harmful preservatives. It could be better replacement for the chemical plant protectants to protect the post harvest products. The environmental degradation could be retarded to some extent as earth and its biome is at serious threshold of deterioration. All countries must heed the warnings of the ‘union of concerned scientists’ to protect the earth for future generations.14‒22
None.
The authors declare that there is no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.