eISSN: 2575-906X


Research Article Volume 2 Issue 6
Department of Biology, Universidad del Valle, Colombia
Correspondence: Alexander Mora Collazos, Department of Biology, Universidad del Valle, Cali, Colombia
Received: December 24, 2017 | Published: November 13, 2018
Citation: Collazos AM. Electrogenic activity and hexavalent chromium reduction by Aeromonas hydrophila CrMFC5. Biodiversity Int J. 2018;2(6):518-522. DOI: 10.15406/bij.2018.02.00106
One of the main challenges for today's science is to decrease the dependence on the use of nonrenewable energy and the decontamination of natural environments. In this regard, microorganisms could play an important role, since their great capacity for metabolic adaptation, high phenotypic plasticity and rapid reproduction, are key elements in the search of solutions of the previously mentioned problems. The current study evaluated the potential for the reduction of hexavalent chromium associated to biological activity and enzyme activity of chromate reductases using cell-free extract of the electrogenic and chromium resistant strain Aeromonas hydrophila CrMF5. Analysis of the 16S rRNA gene placed the isolate close to the speciesA. hydrophila. Strain CrMF5 tolerates a chromium concentration of approximately 360mg/L; in the microbial fuel cells, this organism showed electrogenic activity by registering a maximum power density of 7.1mW/m2. 100% reduction of Cr(VI) in 10mg/L bioassays that was reached at 10 hours in LB medium and at 38 hours in industrial wastewater. The cell-free extract showed Cr(VI) reduction activity with optimum values with a temperature of 32ºC and pH of 6.0, in the presence of NADH. The results suggest a good potential for the production of electrical energy in microbial fuel cell and bioremediation processes of water contaminated with hexavalent chromium, particularly when concerning detoxification of industrial wastewater.
Keywords: Aeromonas, cell-free extract, Cr (VI) reduction, microbial fuel cell
Chromium is a metal of transition with multiple states of oxidation from -II to VI, being the trivalent chromium, Cr(III) and hexavalent chromium, Cr(VI) the most common, stable and biologically important states.1 The different oxidation states of chromium confer it different chemical properties, degrees of toxicity and mobility in natural environments.2 Hexavalent chromium is one of the most hazardous metal ions that is released into the environment by electroplating, leather tanning, mining and metal finishing industries, amongst others.
Cr(VI) exhibits a much greater solubility, mobility, bioavailability, and toxicity than Cr(III). The Cr(III), is stable in natural environments, presents a low solubility through biological membranes and precipitates, is quickly absorbed by suspended matter or sediments.3 In contrast, Cr(VI) is found principally soluble in aquatic environments, is extremely toxic to living organisms causing allergies, irritations and respiratory track disorders and it is considered mutagenic and carcinogenic agent.4 This toxic action is due to the fact that Cr(VI) complexes can easily penetrate cellular membranes and undergo immediate reduction reactions, leading to the formation of various reactive intermediates that cause damages to DNA and alterations to biological functions.3,5,6
A wide variety of Chromium resistant bacteria have been reported for reducing Cr(VI) to Cr(III).3,7,8 It has also been demonstrated, that the prokaryotes possess high metabolic plasticity, rapid adaptation and mechanisms that confer them greater resistance to the toxic effects of Cr (VI) in comparison to eukaryotes.9 The use of Cr(VI)-resistant bacteria for the detoxification of Cr(VI) has been considered an economical, effective and safe procedure over physical and chemical conventional methods.9,10
There are few reports that indicated biological reduction of Cr(VI) by representatives of genus Aeromonas [33], some of these indicated that Aeromonas exhibited poor chromate-reducing activity.11 Representatives of the genus Aeromonas have been detected in MFCs anodes,12 showing great electrogenic capacity.13 Microbial fuel cells (MFC) are an emerging biotechnology that allows the conversion of chemical energy contained in organic wastes into electricity while providing a mechanism for simultaneously treating the wastewaters containing pollutants, using microorganisms. This treatment uses the microbiologically catalyzed reduction of Cr(VI) to Cr(III) in MFCs, process which has recently gained extensive attention.14‒16 By understanding the importance of the biological component in the development of this biotechnology, this study sought to contribute to the knowledge of electrogenic microorganisms with the ability to reduce chromium.
The bacterial isolate CrMF5 was previously isolated from the anodic biofilm placed in sediment from a microbial fuel cell operated with lake sediments.
Characterization and identification by 16S rRNA gene
The molecular characterization of bacterial strain CrMFC5 was performed; using the Ultra Clean Microbial DNA Isolation Kit (MOBIO LAB), extraction genomic DNA was performed. The extracted DNA was amplified using primers: 27F 5’AGAGTTTGATCMTGGCTCAG3’ and 1492R 5’ TACGGYTACCTTGTTACGACTT3’. The sequence was determined by standard procedures, using the service provided by the Macrogen Company (USA). The sequence obtained was deposited in the GenBank database with the accession number: KU724074.
16S rRNA partial gene obtained was compared with sequences deposited in the GenBank using the tool BLAST available on NCBI website and Classifier tool available on the Ribosomal Database ProjectII website.17 Sequences were collected from the different species of the genus that were related to the 16S partial sequence obtained from the CrMF5 isolate. Nucleotide sequences were aligned using ClustalW as implemented in the BioEdit software.18 The phylogenetic tree was inferred by PAUP, version 4.0b10 (maximum likelihood method), the nucleotide substitution model was selected by Akaike criterion in the ModelTest, version 2.1.319 and statistical support was evaluated by means of the bootstrap of maximum likelihood with 1000 repetitions.
Electrochemical characterization of bacterial isolated CrMFC5
The electrochemical performance of the bacterial isolated CrMFC5 was evaluated in a microbial fuel cell (MFC). Single-chamber microbial fuel cells were constructed using 100ml containers. Electrodes were constructed using graphite rods with a surface area of 11.3cm2; for the cathode, the electrode was coated with platinum-carbon catalyst with a concentration of 0.1mg/cm2 prepared in a solution of 5% Nafion. The MFC was kept in operation during 15 days, time in which the electrochemical performance of the microorganism was monitored. Voltage and current were taken using digital multimeters (EX505 EXTECH). The data that was obtained was used to construct power density curves versus time.
Minimum inhibitory concentration assay
Bioassay of hexavalent chromium reduction
The potential of Cr(VI) reduction and the growth kinetics of the Aeromonas CrMFC5 was evaluated in Luria Bertani broth (LB) and industrial wastewater (IW), adjusting the concentration of Cr (VI) to 10mg/L in both cases. The growth kinetics was estimated by optical density (OD) at 550nm. Cells were harvested (8000rpm for 5min) and the obtained supernatant was used for chromium estimation. Hexavalent chromium was determined colorimetrically using the 1,5-diphenylcarbazide (DPC) method. Cr(VI) in the sample was assayed by adding 200µl of DPC reagent, 40µl of H2SO4 (5M) and 500µl of chromium samples to 9.26ml of distilled water, mixed gently and kept at room temperature for five minutes. The absorbance was measured at 540nm. The Cr(VI) concentration in the samples were calculated from a standard curve using K2Cr2O7 as standard.
The IW was obtained from an electroplating company, using the wastewater produced after passing through the retention tanks and before being discharged as waste. The waste had a pH of 5.5 and contained nickel (14.11mg/L), chromium (10.45mg/L) and zinc (0.67mg/L). The IW was supplemented with glucose (5mM) and sterilized by filtration.
Cell-free extract preparation
The CrMFC5 isolate was grown overnight in LB medium, with 20mg/L of Cr(VI). The cells were harvested by centrifugation at 4000 rpm for 10 min at 4ºC, washed two times and re-suspended in phosphate buffer (0.1M; pH 6.5) in a volume equivalent to 10% of the original culture and kept in an ice bath. Cells were lysed with an ultrasonicator (five cycles of 59s on and 59s off at 130watt) (Sonics, Vibra Cell Ultrasonic Processor VCX-750). After sonication, the suspension was centrifuged at 12000g for 10min at 4ºC. The supernatant that contained cell-free extract (CFE) was collected and used as crude chromate reductase enzyme.21,22
Chromate reductase assay
The reaction mixture (1ml) contained Cr(VI) (5mg/L), 0.1mM of NADH as an electron donor in 800µl of phosphate buffer (0.1M; pH 6.5) and 200µl of CFE. A mixture with similar composition without enzyme was used as control sample. The reduction of Cr(VI) was measured by estimating the decrease of Cr(VI) in the reaction mixture after 30min of incubation and colorimetrically quantified using DPC as the complexing reagent.21,23
Effect of pH and temperature on chromate reductase activity
The optimum pH and temperature for the activity of the chromate reductase present in the CFE was determined by incubating the reaction mixture at different pHs using various phosphate buffers (0.1mM, pH 5.5, 6.0, 6.5, 7.0 and 7.5) at 32ºC and different temperatures ranging from 28 to 40ºC at a pH of 6.5. The enzyme activity was determined after 30 minutes of incubation by the DPC method. Relative activity was expressed as a percentage of maximum activity taken as 100%.
Strain CrMFC5 is a facultative and Gram-negative bacilli microorganism that formed circular cream colored and flat colonies; presented smooth surface creamy consistency; bright colony. It showed resistance to Cr(VI) with a MIC value of 350mg/L.
Molecular characterization
The naive Bayesian classifier tool was used to compare the sequence obtained to the sequences deposited in Ribosomal Database Project, indicating that said sequence belongs to the genus Aeromonas with a significance value of 100%. Figure 1 shows the phylogenetic analysis of the sequence of the isolated CrMFC5 with different sequences of species from the genus Aeromonas represented by Maximum likelihood tree based on the GTR (I+G) model. This model was used with estimated values for the following parameters: base frequencies (A=0.2465, C=0.2276, G=0.3257, T=0.2002), the proportion of invariable sites (0.4820), and the gamma-shaped parameter (0.4040). On the phylogenetic reconstruction based on the 16S rRNA gene sequence, strain CrMFC5 was assigned to the species Aeromonas hydrophila, which is included in the group Proteobacteria, subgroup Gamma, and in the family of Aeromonadace.
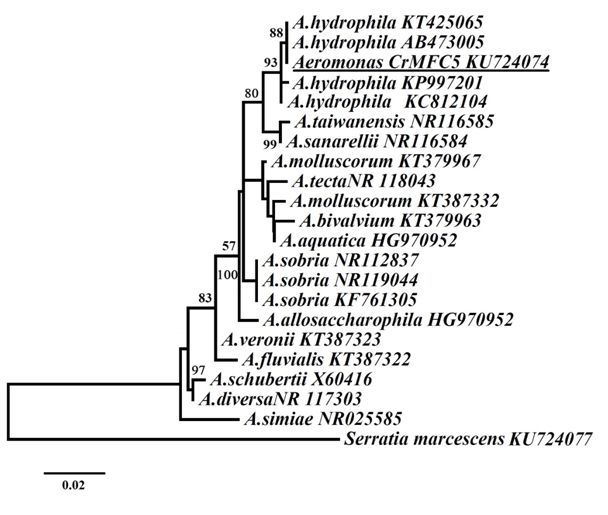
Figure 1 Maximum likelihood tree of the genus Aeromonas based on the 16S rRNA gene, representing the phylogenetic inference of the sequences. Numbers at nodes represent bootstrap values.
Electrogenic activity of Aeromonas CrMFC5
The electrogenic activity of A. hydrophila CrMFC5 is shown in Figure 2. The phase of latency or colonization of the anode took place before day two, showing low values of power density (0.026mW/m2). Subsequently, an increase of values was recorded, up to a maximum value in power density of 7.1mW/m2. The behavior of the data and the registered power density values indicate that A. hydrophila CrMFC5 is an electrogenic microorganism.
Biological reduction of hexavalent chromium
Chromium reduction and the kinetics of the bacterial growth of A. hydrophila CrMF5 in LB broth and industrial wastewater are shown in Figure 3. 100% reduction of Cr(VI) was registered in the LB broth in 10 hours, with an average rate of reduction of 1mg/L per hour. The bacterial growth in LB medium kept increasing constantly, showing a maximum value of absorbance of 0.856 at the end of the test. In contrast, 100% reduction of Cr(VI) in the IW was registered in 38 hours, with an average rate of reduction of 0.31mg/L, indicating a 68% decrease in the average rate of Cr(VI) reduction in contrast to that in the LB medium. The kinetics of growth in the IW were different from those in the LB medium, showing a slight increase in the first 10 hours of the test with a maximum absorbance of 0.495 at the 10th hour.
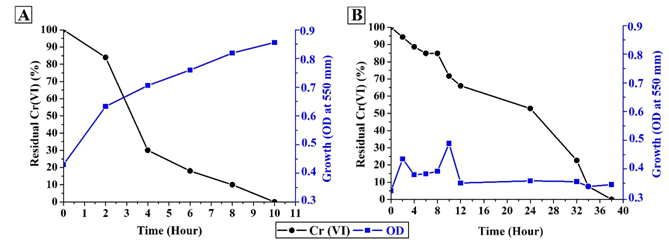
Figure 3 Chromium reduction and kinetics of bacterial growth. (A) Luria Bertani broth. (B) Industrial wastewater.
Chromate reductase activity of cell-free extract
The cell-free extract of A. hydrophila CrMFC5 showed Cr(VI) reduction activity. The optimal temperature of the CFE Cr(VI) reductase activity by CFE was determined by using different incubation temperatures between 28 to 40ºC. Maximum Cr(VI) reductase activity was observed at 32ºC (Figure 4). Activity at 30, 34, 36 and 38ºC was about 54% of the optimal activity. Activity at 28 °C was 64% of the optimal activity and at 40ºC, the activity was at 45% of the optimum.
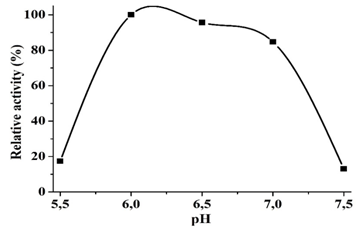
Figure 4 Effect of pH on Cr(VI) reduction activity by CFE of the bacterial strain A. hydrophilaCrMFC5. The reductase activity was determined after 30min of incubation at 32ºC.
The effect of pH on reductase activity of CFE was assessed at a pH range from 5.5 to 7.5, using phosphate buffer. Significant values of Cr(VI) reduction activity were observed over a wide pH range. At pH 6 and 7, the Cr(VI) reduction activity shown was of 95 and 84% respectively, with an optimum at pH 6.0 (Figure 5). An observed decrease in chromate reducing activity was similar towards both alkaline and acidic conditions. The chromate reducing activity diminished rapidly prior to a pH of 6.0 and after a pH of 7.0, showing a 17 and 13% of the optimal activity, respectively.
Phylogenetic analysis revealed that the strain CrMFC5 is included in a group comprehended by Aeromonas hydrophila. In this document, we described a new Cr(VI) resistant and Cr(VI) reducing strain, A. hydrophila CrMFC5. The genus Aeromonas is a group of ubiquitous microorganisms found in a wide range of aquatic environments24 and according to scientific literature, these species of bacteria are rarely known by their electrogenic capacity and Cr(VI) reduction.
The power density data registered by A. hydrophila CrMFC5 (7.1mW/m2) is low in comparison to other authors.25‒28 Notwithstanding, these low values reported were a product of the materials used in the construction of the electrodes and the small available surface area for biofilm formation. The obtained results allow for the classification of this microbial isolate as an electrogenic strain with a higher potential than the one shown in this paper. Representatives of the genus Aeromonas have been detected in MFCs anodes,12 showing great electrogenic capacity.13 The Nanowires detection in representatives of the genus Aeromonas,29 indicates its possible mechanisms of electron transfer and confirms the electrogenic potential that these microorganisms possess.
The rate of chromium reduction and microbial growth was completely different when Aeromonas CrMFC5 was evaluated in LB and IW. In the LB broth assay, the Cr(VI) reduction rate didn’t seem to be influenced directly by the increase in the number of cells. The highest rate of reduction was registered between 2-4 hours with a 54% reduction of the total of Cr(VI). In the following hours (4-6, 6-8 and 8-10) a rate of reduction of 12%, 8%, and 10% respectively, of the total of Cr(VI) was registered. It was possible to observe a decrease in the rate of reduction of chromium, although an increase in the number of microorganisms was presented (Figure 3). Similar results were observed in the work of Dey et al.30 who tested four different bacterial strains, showing that the highest rate of reduction was observed in the first hours of the assay. In contrast, in the assays performed with IW a significantly lower growth was showed than the one observed in LB. The average rate of Cr(VI) reduction per hour during the first 10 hours of the assay was 57%. However, the average rate of Cr(VI) reduction per hour during the last 10 hours of the assay was of 1.94% of the total of Cr (VI). The behavior that was observed with the IW differs completely from the one observed with LB medium.
The Cr(VI) reduction bioassays realized in rich mediums such as LB allow a rapid determination of the presence of microbial mechanisms that would enable them to reduce chromium. Notwithstanding, the differences in the kinetics of growth and the Cr(VI) reduction between the different bioassays (Figure 3), indicate that in a rich medium it’s not possible to obtain real inferences on the bioremediation potential that a microorganism may have.
With the objective of simulating the conditions of a wastewater treatment, the characteristics of the collected IW were not altered, indicating that the A. hydrophila CrMF5 strain has the potential to efficiently reduce Cr(VI) in the presence of other metals such as Ni (14.11mg/L) and Zn (0.67mg/L), and at pH of 5.5. Representatives of the genus Aeromonas with the ability to tolerate nickel,31 the potential to reduce iron32 and to tolerate cadmium, copper, lead and mercury33,34 have been reported. The genus Aeromonas and strain CrMFC5 could to be promising and useful in the treatment of industrial wastewater contaminated with Cr(VI) and other metals. The enormous potential for biotechnology and bioremediation using microorganisms present in the genus Aeromonas is still unknown.29 Bacteria resistant to heavy metals in MFC anodes could represent an important opportunity for the treatment of industrial or domestic wastewaters with a high concentration of heavy metals and/or organic matter, which currently poses a serious environmental problem.
This study revealed that A. hydrophila CrMFC5 has enzymes with chromate reductase activity. In the CFE assay, NADH was used as an electron donor, since previous reports indicated an improvement in the presence of NADH.21,22,35
The Cr(VI) reduction activity of the CFE achieved a maximum value at 6.0 pH (Figure 4). The optimum temperature for the highest value of Cr(VI) reduction was found at 32ºC (Figure 5). These results vary from other earlier reports; in the case of Halomonas sp. TA-04, the optimal were found at 28ºC and a pH of 6.5.21 A. rhombi showed optimal values at 30ºC and a pH of 5.5.36 Between a pH of 6 and 7, the CFE of A. hydrophila CrMF5 showed a relative activity greater than 80% of the maximum reduction activity with an optimum value at pH 6.0. Exiguobacterium sp. KCH5 also shows an optimum value at pH 6, and a broad range of relative activity greater than 80% of maximum reduction activity from pH 5.0 to 6.5.37
The rate of degradation of pollutants by microorganisms can be limited by several environmental factors such as temperature, pH, competition with other microorganism and by other pollutants present in the same environment.8,38 In this sense, the use of enzymes isolated from bacterial could be more advantageous than using whole microorganisms.39 Sandana-Mala et al.40 demonstrated the feasible bioremediation process by enzymes using a crude extracellular enzyme to reduce chromate in tannery effluent. The sole use of enzymes could be more favorable when environmental conditions are unfavorable for the microorganisms.
The isolated bacterial strain from the genus Aeromonas tolerated Cr(VI) and reduced Cr(VI). In addition, the CFE has enzymes with chromate reductase activity. The ability of the isolated strain to reduce Cr(VI) in industrial wastewater provides the opportunity for bioremediation of industrial water containing heavy metals. Moreover, the use of CFE could represent a further opportunity for Cr(VI) reduction. The electrogenic stain A. hydrophila CrMFC5 would provide the opportunity to treat Cr(VI) from wastewater and to simultaneously generate electricity. We suggest the further exploration of the potential of the microorganisms belonging to the genus Aeromonas and use of the new isolate in bioremediation applications.
The research group “Biology of Plants and Microorganisms” and the Laboratory of Microbiological Investigations of the Department of Biology; the “Laboratory of Biofuels” of the School of Chemical Engineering of the Universidad del Valle (Cali, Colombia); the Organization Idea Wild (USA) for the donation of equipment required for the measurement of electrochemical parameters and the company BYCSA S.A for the donation of the industrial wastewaters.
There is no conflict of interest to declare regarding the publication of this paper.

©2018 Collazos. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.