Advances in
eISSN: 2572-8490


Research Article Volume 9 Issue 1
Correspondence: Laila Mahmoud Montaser, Professor of Clinical Pathology, Chair Stem Cell, Regenerative Medicine, Nanotechnology and Tissue Engineering (SRNT) Research Group, Egypt
Received: September 02, 2023 | Published: October 3, 2023
Citation: Montaser LM. A prospective perspective on the advances in robotic- assisted 3D bio-printing for tissue engineering. Adv Tissue Eng Regen Med Open Access. 2023;9(1):35-40 DOI: 10.15406/atroa.2023.09.00139
3D printing involves cutting through the middleman. This advantage is transferred to in vitro bioprinting to cut the middle step of in vitro cell growth in the lab and transplant stem cells directly into the body for growth (in situ). On-site bioprinting with robots can be used for some surgical procedures because they are less invasive to the patient, with minimum pain, lesser recovery time, less opportunity of infection, and smaller time spent in hospital. Expect the use of mobile emergency printers in developing countries and remote areas. This was the brief summary of my invited speech which was sent to Global TIPE 3D printing conference 2022, Jan 18-20, New York, USA organized by Women in 3D printing located in New York City, NY, USA titled "Modern technologies/Where are we leaving?", the target of these talks was to give insight, best practices, or strategic information to the audience. My invited talk showed in the 1st day of the Conference awarded a certificate of appreciation*(Figure 2c) for successfully presenting my research/academic work. The awards are a great chance for scientists to celebrate their success and grace people whose realizations are a model for young people to walk behind. (Scheme 1)
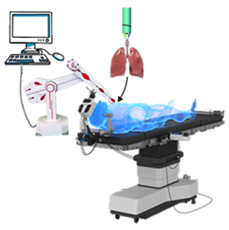
Scheme 1 Diagram of a graphic simplified summary of the notion of robotically assisted in situ 3D bioprinting for less invasive surgery.
Keywords: in situ 3D printing, robotic-assisted bioprinting, stem cells
We are stamped off 2022 with high prospects for an outlook of fabulous outstanding scientific detections in 2023, increased potential in sustainability, and the future innovators of scientific generations. My aim is to present a viewpoint that sheds light into future advances in robotics-assisted in situ bioprinting for tissue engineering and regenerative medicine.
Tissue defects resulting from disease, trauma or surgical removal demand cure to retrieve tissue structure and function. There are some troubles related to the reconstruction of complex tissue defects, such as those affecting the skeleton or cranial and facial area and those resulting after surgical degradation. Such defects are difficult to remedy with conventional and standard size implants. In particular, they need treatment using modified or manicured implants or formulations. 3D printing provides the possibility of providing fixtures that can be pre designed to fit the size and shape of the defect accurately. These formulations are usually designed and engineered outside of the vivo and are then transplanted into the body. However, this process has many challenges related to logistics (3D printing in a place far from the operating room), sterilization, and the need to modify, cut, adjust or combine different pieces to suit the shape and size of the handled defect. In addition, the size and shape change and therefore the ready printed structure may not always fit after the trouble is detached. Alternatively, the 3D implant can be installed in a subsequent second process and this two-stage procedure involves greater risks and problems. Ideally, a custom implant should be produced and instilled in the same setting. However, this approach has been constrained by many technical, procedural and organizational issues to date. This ideal solution will use a commonly used 3D printing technology in the operating room and on-site 3D printing constructions. This concept may involve the use of any of the portable printers1 or robotic arms carrying printer nozzles, which are controlled by computers and scanners, which constantly measure the exact size of the defect. Integration of developments in robotics and computer-assisted interventions will also enable greater accuracy in printing procedures. These developments will allow the development of more innovative solutions for on-site 3D printing for use in operating rooms. When fully deployed, the on-site 3D printing technology will lead to a more accurate reconstruction of tissue defects and lead to faster and more efficient healing of tissue defects.2
3D bioprinting is an emerging bioprinting technology, driving many innovations and opening up new ways in regenerative treatments. The goal of 3D bioprinting is to manufacture grafts in the lab, which can then be implanted in the vivo. However, transplantation of tissue outside of vivo carries safety risks, and therefore complex manufacturing equipment and practices are required to transplant tissue in humans. Instilling printed tissue also adds complexities due to the difficulty of maintaining the structural integrity of manufactured formulations. To meet this challenge, the notion of 3D bioprinting has been proposed on site where tissue is printed directly at the site of injury or defect. This approach can be combined with newly isolated cells from patients to produce customized grafts that resemble the target tissue and accurately fit the target defects. Furthermore, the body's natural cellular environment can be harnessed to mature tissue leading to tissue renovation and reform. Tissue engineering methods allow the manufacture of tissue offsets that combine cells, biomaterials and bioactive compositions to exchange or redress injured or diseased tissues. As technology advances on site, it is believed that in the future, a little slice of any tissue can be bioprinted on site, during surgery, in short and exactness.
Laila M. Montaser viewpoints on application of 3D bioprinting technology
Laila M. Montaser stated in Jan 20233 that present technology is incapable to make copied tissue-engineered structures with the equivalence of the natural tissue. The modern development of 3D printing technology enables the fabrication of tissue substitutes integrating cells, biomaterials, and bioactive compounds and the concept of in situ 3D bioprinting in the lab to cut out the middle step of in vitro growing cells and implanting cells directly into the body to repair damaged tissue or organ via tissue engineering. This type of technology is less invasive, offers great advantages to the patients and hopes to offer a new solution for those on the waiting list.
Prof. Laila Montaser announced in August 20224 that the coronavirus is one of the major human challenges of modern times. The human brain is planned to favor the present and reduce the future. This makes it difficult to prevent calamities such as pandemics. Drug numbers are currently being tested for COVID-19. While these curatives can improve the patient's recovery and presence, these therapeutic methods do not explicitly repair the lung injury caused by this disease. Stem cell treatments are prominent as new favorable treatments, which can reduce inflammation but also regenerate lung damage caused by COVID-19. Stem cells expand their immune-modifying, antioxidant and therapeutic effects that can be useful, individually or combined with other medical factors, in patients with COVID-19. Stem cells can be used to illustrate this harm using their regenerative properties, allowing them to explore the enhanced clinical benefits contracted to examine drug treatments. Recent revenues in regenerative medicine confirmed the opportunity to create viable and effective three-dimensional tissue engineering containing vibrant cells to repair and increase tissue. 3D bioprinting has emerged as a new and hopeful tactic in making complex biological manifestations in tissue engineering and regenerative medicine. It aims to reduce the obstacles of counterfeit tissue engineering procedures by meticulously assembling and striped layer after layer of biomaterials in a three-dimensional manner required. Cellular printing aims to transfer active cells in a three-dimensional style to represent stem cell outlets and pathological tissue forms for drug screening, or to mimic human tissue jamming that acts as a biologically relevant alternative. The flexible 3D tissue engineering technique allows the division of stem cells created from a person's body after printing and discrimination in a course of formation and replacement of any type of body tissue. 3D printing techniques can distinguish stem cells into lung cells. 3D printing can be used to overcome the lack of individual defensive tools caused by the new COVID-19 as well as to employ technology to create samples of human organs and tissues for trial targets. Figure 1 summarizes the 3D bioprinting process: Isolation and proliferation of stem cells utilizing bioactive factors, blending of hydrogel with cells, eclectic sedimentation of "bioactive inks" of bioactive ingredients to fill the dysfunction area with proper geometry, bioprinting on scaffolding in the laboratory, and lastly implantation in the living body to redress the affected region.
Montaser stated also in August 20225 that bioprinting has provided many advantages to traditional tissue engineering methods for scaffolding manufacturing for organ/tissue regeneration. However, this technique, also known as in vitro bioprinting, suffers from many limitations when considering its clinical application, such as the difficulty of handling the scaffolding, the risk of contamination, the need for maturity period in the bioreactor, and the form/formation of bioprinted construction is not ideal matching with the fault site. For these reasons, in situ bioprinting has emerged as a promising alternative technology. The author's goal was to report on-site robot-assisted 3D bioprinting technology for tissue regeneration. In situ bioprinting consists of direct deposition of biological material in the patient, following the complex engineering of anatomical defects to achieve the desired mechanical properties suitable for tissue regeneration. This approach ensures enhanced maturity and differentiation between bioprinted formulations, as the patient's own body acts as a bioreactor. 3D bioprinting can be a promising technique for generating engineered functional tissue. This technique allows for the possibility of repairing defects of complex forms in a short time. Printing devices directly on organs (in a minimally invasive way) can help reduce the need for transplant surgery - as long as there is a smooth integration between printed material and biological materials within the body. 3D manufacturing of living tissue during the process can be the next biomedical revolution in the treatment of patients.
Montaser reported in April 20226 that tissue defects resulting from disease, trauma or surgical removal require treatment to restore tissue structure and function. There are some challenges related to the reconstruction of complex tissue defects. Such defects are difficult to remedy with conventional and standard size implants. They need to be treated with modified or manicured implants or formulations. Instilling printed tissue also adds complexities due to the difficulty of maintaining the structural integrity of manufactured formulations. To meet this challenge, the concept of 3D bioprinting has been proposed on site. A triumphant goal was to manufacture grafts in the lab and then implant them in the vivo. 3D bioprinting on the site in which tissue is printed directly at the place of injury or defect along with newly isolated cells from patients is to produce customized grafts that resemble the target tissue and accurately fit the target defects. The structures were designed, engineered outside the living body and later implanted into the body. This new research field offers great potential for tissue engineering and regenerative medicine applications where it is difficult or impossible to predict pre-surgical planning of the size and shape of the construction. 3D printing can provide fixtures that can be pre-designed to fit the size and shape of the defect accurately. This ideal solution will use a commonly used 3D printing technology in the operating room and on-site 3D printing constructions. When fully deployed, the on-site 3D printing technology will lead to a more accurate reconstruction of tissue defects and lead to faster and more efficient healing of tissue defects. Advances in robotic surgery, compact imaging, and computer-assisted medical interventions should be integrated to develop future clinical 3D bioprinting processes on site, which can be translated into products for a variety of surgical applications.
Montaser explained in January 20227 that 3D printing is about cutting out the middleman. This benefit is transferred to in vitro bioprinting to cut the middle period of in vitro cell growth and transplant stem cells directly into the body for growth (in situ). On-site bioprinting with robots can be used for some surgical procedures because they are least invasive to the patient, with not so much pain, lesser recovery time, minimal chance of infection, and reduced time spent in hospital. Anticipate the use of mobile printers in emergencies and in developing countries and remote areas that show their usefulness and utility. To date, research in the field of 3D bioprinting on site has shown feasibility and avail. This new research domain offers great potential for tissue engineering and regenerative medicine applications where it is difficult or impossible to predict pre-surgical planning of the construction form. The author predicts that this new technology will find interest in various biomedical areas in the near future.
Montaser declared in November 20208 that tissue engineering techniques enable the manufacture of tissue substitutes that integrate biological cells, materials and bioactive compounds to replace or repair damaged or diseased tissue. Despite early success, the current technology is unable to manufacture replicable tissue formulations with structural and functional similarity to the original tissue. The recent development of 3D printing technology offers opportunities for the development of functional complex tissue alternatives through the manufacture of layer by layer of cell(s), bioactive material(s) and compound (s) bioactive with precision and a three-dimensional concept on site. In vitro bioprinting is to cut the intermediate part of developing cells in the laboratory and transplant cells directly into the body for growth. Mesenchymal stem cells (MSCs) have been reported as promising treatments for lung disease. 3D bioprinting assumes human MSCs with bioink hypothesis as a potential treatment for lung damage. Human lungs do not have the ability to regenerate. Building an artificial lung to replace the sick lung is the only alternative to treating patients with acute lung disease. 3D printing helped manufacture the lung tissue isotope. Therefore, clinical advances in the use of bioprinted 3D cancer stem cells as cellular therapy for acute respiratory distress syndrome may have clinical effects during the COVID-19 pandemic. The development of 3D printed biological tissue to replace organs hopes to provide a new solution for patients in the queue. 3D bioprinting is one technique that allows the preparation of alternatives specifically designed for tissues. Rapid prototypes create possibilities for generating complex organs such as kidneys, liver, lung or even heart, although there is a heterogeneous cellular composition. 3D bioprinting approaches can be used to create a model for lung disease and lung tissue. The current development of the manufacture of tissue engineered formulations using 3D bioprinting technology is essential for potential biomedical applications such as tissue replacement therapy, personalized therapy, and laboratory 3D modeling for drug detection(Figure 2).

Figure 2 Some Certificates of Appreciation awarded to Prof. Laila M. Montaser for her keynote speeches on application of 3D bioprinting technology presented in the era of COVID-19: (a) Keynote speech awarded a Certificate of Recognition: Robotic in situ 3D bioprinting technology for the generation of functional engineered tissues. Int. Webinar on 3D printing & additive manufacturing, 2022, Aug 22-23, Singapore city, Singapore. (b) Keynote speech awarded a Certificate of Recognition: In Situ 3D Bioprinting for Tissue Engineering. Int. Webinar on 3D printing & additive manufacturing, 2022, April 13-14. (c) Invited speech awarded a Certificate of Appreciation*: Modern technologies/Where are we leaving? Global TIPE 3D printing conference 2022, Jan 18-20, New York, USA.
Montaser announced in February 20229 that drug numbers are currently being tested for COVID-19. While these treatments can improve a patient's recovery and presence, these therapeutic methods do not explicitly repair the lung injury this disease has caused. Stem cell treatments are prominent as new favorable treatments, which can reduce inflammation but also regenerate lung damage caused by COVID-19. Stem cells expand their immune, antioxidant and therapeutic effects that can be useful, individual or combined with other medical factors, in patients with COVID-19. Stem cells can be used to illustrate this harm using their regenerative properties, which allow them to explore the enhanced clinical benefit that is contracted to examine drug treatments. Recent revenues in regenerative medicine confirmed the opportunity to create a viable and effective 3D tissue engineering containing vibrant cells to reshape and increase tissue. 3D bioprinting has emerged as a new and hopeful tactic in the manufacture of composite biological breeding in the field of tissue engineering and regenerative medicine. It aims to reduce the obstacles of counterfeit tissue engineering procedures by meticulously assembling and layering after layer of biomaterials in a 3D manner required. Cellular printing aims to transfer active cells in a three-dimensional mode to represent stem cell outlets and pathological tissue forms for drug screening, or to mimic human tissue jamming that acts as a biologically relevant alternative. The flexible 3D tissue engineering technique allows the division of stem cells created from a person's body after printing and discrimination in a course of formation and replacement of any type of tissue in the body. 3D printing techniques can distinguish stem cells in lung cells. 3D printing can be used to overcome the lack of individual defensive tools caused by the novel COVID-19 and similarly to employ technology to create samples of human organs and tissues for experimental goals.
Scientists insights into 3D bioprinting
Li and colleagues reported in 202110 repairing long-cut bone defects by bioprinting 3D on site using a 3D printer with an automatic manipulator in a pig model. They systematically improved the vital ink gel under physiological conditions to achieve the desired mechanical properties suitable for bone regeneration, and the D-H motor model was used to improve printing accuracy to 0.5 mm. These technical improvements allowed repairing long sectional defects created on the right leg of pigs using 3D bioprinting within 12 minutes. The 3D bioprinting kit showed improved therapeutic effects after 3 months.
Ma et al.11 proclaimed in 2020 that the concept of 3D bioprinting was previously reported on site, while its investigation continues to face many difficulties. Their study aimed to report 3D bioprinting technology with on-site robot help to regenerate cartilage, and explore its potential in clinical application. A six-degree robot of freedom (6-DOF) was introduced in this study. The experiment was conducted in vivo on rabbits to assess cartilage processing capacity. According to their results, the robot's accuracy can be significantly improved, and the printed surface error was less than 30 micrometers. The cartilage bone dysfunction can be repaired during approximately the 1960s, and the regenerative cartilage in hydrogel implantation and bioprinting 3D kits in situ showed the same biomedical and biochemical performance. They found that cartilage injury can be treated using this method. 3D bioprinting on site with the help of the robot is very suitable for improving surgical procedures, as well as promoting cartilage regeneration. This study indicated the usefulness of this technology for clinical application.
Dr. S. Singhs' team reported also in 202012 that bioprinting techniques have evolved in the convergence of automation, digitization and new tissue engineering approaches. Bioprinting on site can be preferred during certain situations when compared to traditional bioprinting in vitro when de novo tissue is printed directly on the intended anatomical site in vivo. So far, few attempts have been made to manufacture tissue on site, which can be stopped and safely installed during printing in preclinical living models. The authors explained the need and usefulness of on-site bioprinting in relation to traditional bioprinting approaches. The two main on-site bioprinting approaches, the robotic arm and handheld approaches, have been identified and distinguished. Various studies involving on-site manufacturing of skin tissue, bone and cartilage have been clarified. Bioprinting on site may be preferred during certain situations when compared to traditional bioprinting in vitro when tissue is manufactured or repaired directly on the intended anatomical site in vivo, using the body as a bioreactor. However, technology requires further improvement to manufacture complex tissue on site, which can ultimately be possible through interdisciplinary innovations in tissue engineering. This study explains the need, utility and current methods through portable and robotic patterns of on-site bioprinting. In short, recent on-site bioprinting studies have appropriately accommodated the very conceptual idea of bioprinting tissues directly on the live body. Due to its inherent advantages such as using the body as a bioreactor and eliminating the risks associated with manual transplantation of prefabricated formulations, new attempts are constantly being made to develop on-site bioprinting technology.
Murphy and Anthony reported in 201413 that the ideal material properties for bioprinting the choice of materials suitable for use in bioprinting and its performance in a particular application depends on many features:
Campbell and Weiss pronounced in 200714 that tissue engineering augurs well for the production of revolutionary new treatments for tissue and organ regeneration. This emerging field is very broad and selective in its different curricula. However, all strategies being developed are based on the therapeutic delivery of one or more of the following types of tissue building blocks: cells; Matrices or extracellular scaffolding; hormones or other signaling molecules. To date, most work has used mainly homogeneous combinations of these ingredients, with subsequent self-regulation to transfer a certain level of tissue function that occurs during laboratory culture or after transplantation. The emerging "bioprinting" methodologies for the creation of tissue engineering formulations are initially investigated with more specific spatial regulation, driven by the hypothesis that biomimicry patterns can produce improved therapeutic results. Inkjet-based bioprinting and related printing techniques can be used to manufacture continuous biomimicry patterns that can be used to study the basic biology of tissue regeneration and possibly translate into effective clinical treatments. However, recapitulating nature at even the most primitive levels such as integrating printed cells, extracellular matrices and hormones into hierarchical and spatially organized 3D tissue structures with appropriate functions remains a major challenge.
Mironov and his team stated in 201115 that organ printing, or layer after layer, robotic biomodulation of 3D functional tissues and organs using spherical building blocks of self-assembling tissue, is a rapidly emerging technique that promises to convert tissue engineering into a felicitous biomedical industry trade. It is increasingly clear that analogous decided industries apply to automated systems on the road to trade translation and economic prosperity. However, the harness of robotic bioprinters single is not enough to develop bioprinting of organs on a major industrial domain. Designing and developing a bio-integral manufacturing line for members is essential for the trade interpretation of organ printing technology. This study offers novel advance and oppositions in the evolution of necessary constituents of organ' bioprocessing line. The authors highlighted the following:
Chamitachal and his colleagues defined in 201916 3D bioprinting as an added bioprinting technique with the ability to speed up translated research, as it has the ability to manufacture tissues and synthetic organs that closely mimic biological tissues or organs. As an emerging research area in tissue engineering, 3D bioprinting has a domain in the development of transplantable tissue and organs, building tissue/organ models and high-productivity cancer symbols for pharmaceutical and toxic studies. Moreover, this area has been diversified through the continuous updating of 3D bioprinters and biomaterials, which play key roles in architectural quality and bioprinted construction functions. Addressing these technological complexities requires an integrated approach that incorporates experience from different areas of science and engineering with lateral thinking. Biological tissue replication at the microscopic level ensures successful structural generation of tissue mimicry. Cells in tissues mimic and reshape the extracellular matrix, which in turn regulates cellular movement, growth and differentiation. The extracellular matrix (ECM) also facilitates the microenvironment by harboring soluble agents, chemokines and growth factors. It also provides physical cohesion and anchoring of cells through connections. Bioengineering approaches focus on reproducing these cellular and extracellular components found within tissues to develop tissue repetitions that can be used for the clinical restoration of tissue or organ function. A major challenge in this area is the reproduction of the complex micro-structure of ECM, biochemical agents and their gradients and the presence of multiple cell species in a particular tissue.
Min and his team stated in 202117 that 3D printing, a technique that allows the construction of layer after layer of complex 3D structures from a range of raw materials, demonstrated a promising applicability in construction and in the automotive, aerospace, defense, biomedicine, and consumer electronics industries. However, the main concern is the health effects and safety problems due to the emission of fine particles (PM2.5, particles with aerodynamic diameters <2.5micrometers) and volatile organic compounds (VOCs). Understanding the relevant toxic properties and effects of 3D printing PM2.5 and volatile organic compounds is important for assessing health risks and safe application. At the same time, standard risk assessment protocols for 3D printers are recommended for a better comparison of results.
Bioreactors for organ printing
Yan and his colleagues explained as early as 200518 that bioprinting itself is not enough to create functional tissue or build organs that are immediately suitable for transplantation. It takes time for bioprinted tissue semi-spherical objects and bioprinted tissues to be bioprinted to assemble, compress, reshape, and mature in functional tissue structures. Subsequent treatment may be the most fundamental step in organ printing technology, and effective subsequent treatment or rapid maturity of tissues will require the development of new types of bioreactors. Yan and others announced that they have developed a technique to manufacture organs that enables them to form complex 3D structures of biomaterials/cells in designed patterns. This technology uses a high-precision 3D precision positioning system with pressure-controlled syringe to deposit cell/biomaterial structures with a side resolution of 10 micrometers. The precision syringe activated by pressure is equipped with precision cavity output needle using a variety of 3D patterns with different arrays of channels (through holes). Channels can provide living cells with nutrients and allow the removal of cell metabolites. Compact cells remain viable and perform biological functions as long as the three-dimensional structures are maintained. New technology has the ability to ultimately produce high productivity of synthetic tissue and human organs. The authors concluded that the three-dimensional structures obtained could provide living cells with nutrients and allow the removal of cellular metabolites. Compact cells remain viable and perform biological functions as long as the three-dimensional structures are maintained. This 3D cell assembly technique has raised new horizons in tissue engineering and has the potential to ultimately produce high productivity for artificial tissue and human organs.
Final thoughts and prospects
This perspective is consistent with the explanation of Tripathi and colleagues19 that 3D bioprinting or additive manufacturing is an emerging creative technology revolutionizing the domain of biomedical applications by incorporating engineering, manufacturing, art, education, and medicine. This procedure included integrating the cells with biocompatible materials to plan the required tissue or organ pattern in situ for many in vivo applications. Besides, they added that since the 15th century, printing has been known as one of the most vital processes of manufacturing versions or images for faster and widely knowledge publishing. It is also known as a new and innovative method to change knowledge. It has also had an effect on society by affecting the nation's education, politics, religion, and language. Since 2D printing is a rise-cost technology, raising the time and diminishing the expandability of investing a specific outcome is the request of the hour. These restrictions are conquered by these 3D printing technologies as they assist defeat multiple manufacturing challenges worldwide.
Abdolmaleki et al.20 reported the building of creative 3D scaffolds with complicated structures applying bioprinting to defeat nerve tissue regeneration difficulties. The therapeutic possibility of this method for implementation to both the central and peripheral nervous systems was evaluated. Their research presented a summary of novel improvements in 3D bioprinting advancement and their medicinal possibility for the nervous system.
Forty four certificates of appreciation for unprecedented unique accomplishment were awarded for successfully presenting fifty three global online conferences 44/53 (83%) in recognition of Montaser contributions of the commitment to provide the international community with continuous education from her home office since the start of COVID-19 crisis. For Montaser that feat was the starting of many scientific endeavors that transferred her from researching in experimental animal models in her Faculty lab before the lockdown of COVID-19 crisis until presenting world-class perspectives in possible implementation of Platelet-rich plasma rich in growth factors or execution of stem cells with/or without nanotechnology as a potential treatment for COVID-19 until lastly the idea of application of stem cells with robotic- assisted in situ bio-printing for tissue engineering.
This research introduces the latest bioprinting technology applications in tissue engineering and regenerative medicine. Bioprinting remains a promising solution to address the growing shortage of organs for transplantation globally. The ability is to produce transplant tissue with a less promising immune response risk in synthesis of artificial organs. Recent advances in hydrogel science, including the development of dynamic convertible hydrogels and oxygen that produce hydrogels, provide more and more ways to control the precise environments of the cell. However, the full potential of 3D bioprinting can be achieved through improved printing speed, bioprinting capability of different scales, availability of bioprinting materials and hydrogels, tissue sensitization, tissue dysfunction, on-demand scaffolding manufacturing and cell maturity mechanisms. 3D bioprinting involves printing cells and materials like ECM components together to form a viable textile structure after a one-step bioprinting process. This is followed by the incubation of tissue or 3D-printed organs in a bioreactor before any other experimental procedures are performed and then passed for preclinical and clinical trials.
None.
The author declare that there are no conflicts of interest.
None.

©2023 Montaser. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.