Advances in
eISSN: 2378-3168


Research Article Volume 12 Issue 4
University of Science Arts and Technology, BWI
Correspondence: Orien L Tulp, University of Science Arts and Technology, Olveston, Montserrat, BWI
Received: August 19, 2022 | Published: August 30, 2022
Citation: Tulp OL. The effect of phenotype on development of brown and white adipose tissue cellularity and nonshivering thermogenesis in the wistar fatty rat . Adv Obes Weight Manag Control. 2022;12(4):123-130. DOI: 10.15406/aowmc.2022.12.00374
Brown adipose tissue (BAT) has been noted in cadaveric specimens by anatomists for hundreds of years and is now known to be a primary peripheral tissue in the expression of non-shivering thermogenesis in response to perturbations in diet and environment in homeothermic species including man and animals. The Wistar Fatty Rat (WFR) is an animal model of obesity, insulin resistance (IR) and NIDDM and expresses the (-fa) obesity trait in an NIH/Wistar background. This stain has been reported to exhibit NIDDM and an impaired thermogenic response to parameters of diet and environment in the obese phenotype. Groups of lean and obese male WFR rats were maintained in hanging wire-bottomed steel cages and fed a nutritionally complete diet containing 54% CHO as equal parts cornstarch (ST) and sucrose (SUC) (50:60 w/w) plus vitamins, minerals, fiber, and essential micronutrients from 22 to 30 weeks of age. Measures of body weight were monitored and measures of resting, and norepinephrine stimulated VO2 determined. Animals were sacrificed by decapitation and the Interscapular BAT depot (IBAT) and primary white adipose tissue (WAT) depots excised in their entirety for measures of adiposity including adipocyte size and number per IBAT and WAT depots. The studies included measures of adipocyte lipid content and adipocyte number of WAT depots including the dorsal (DOR), epididymal (EPI) and visceral retroperitoneal (VRP) depots. Final Body weights, net weight gain and relative adiposity of obese were significantly greater than their lean littermates throughout the study with the greatest increase in WAT cell lipid content and adipocyte number in the VRP depot. IBAT cell number, cell lipid content of IBAT tissues and IBAT:BW ratio of obese >> lean littermates. Fasting glucose was similar in both phenotypes, but fasting insulin and the Insulin: Glucose (I: G) ratios were markedly elevated in obese+NIDDM animals. Resting VO2 and the thermic response to NE of lean >> Obese+NIDDM. A robust NE response in plasma glucose concentrations occurred in both phenotypes following NE with the greatest increase in the obese+NIDDM phenotype. The results of this study indicate that while the development of IBAT and WAT mass and cellularity became exaggerated via hyperplasia and hypertrophy in the obese-NIDDM animals, the superimposition of early hyperphagia and the NIDDM stigmata which likely includes the development of significant IR is a contributing factor. The elevations in I: G and IR of the Obese+NIDDM phenotype may further facilitate adipocyte hyperplasia and hypertrophy in both WAT and BAT depots and may further compromise the capacity of the obese diabetic animals to fully express BAT-mediated contributions to NST. Moreover, the increased BAT mass and cellularity in the Obese+NIDDM was not by itself a reliable predictor of the thermic responses to diet and environment.
Keywords: obesity, rat, brown adipose tissue, thermogenesis, norepinephrine, insulin, glucose, glycogenolysis, glycemia
WAT, white adipose tissue; IR, insulin resistance; NST, non-shivering thermogenesis
The observation of what we now recognize to be brown adipose tissue (BAT) appears to have been made by anatomists in human cadavers over 600 years ago and in hibernating animals for nearly 100 years.1,2 BAT is found in numerous homeothermic animals and hibernating species, where anatomically it is strategically located adjacent to critical vasculature such that heat energy that may be generated from the tissue may be efficiently transferred to critical organs and tissues during arousal from states of torpor, hibernation and following perturbations in diet and environment.2–6 Brown adipose tissue is well recognized for its essential role in the expression of non-shivering thermogenesis (NST) in rodents in response to alterations in diet and environment, where its primary function has been proposed to convert stored energy from multiple contained cellular lipid molecules and dietary sources to heat energy that may be subsequently dissipated via close proximity of the BAT depots to nearby vasculature where it may contribute to the maintenance of body temperature regulation and also act as a contributor to energy balance during variations in dietary energy content and macronutrient distribution. The activation of BAT likely begins with signals directed at sensory receptors in the hypothalamus and culminating in direct stimulation of stereospecific β3 adrenergic receptors located in brown adipose tissue which can then bring about the activation and release of uncoupling protein-1 (UCP-1), and the subsequent uncoupling of oxidative phosphorylation of ATP to form ADP and the release of thermal energy released from hydrolysis of the high energy phosphate bonds of ATP.4
The uncoupling protein 1(UCP1) is unique to brown adipose tissue of mammalian organisms and is the key component of the β-adrenergically controlled nonshivering thermogenesis (NST) process in brown adipocytes. The UCP-1 protein that mediates the activation of BAT thermogenesis may be activated following exposure to cold environments or in response to a variety of nutritional factors.2–4 Several authors have reported fundamental aspects of the physiologic and biochemical mechanisms involved in the expression of UCP-1 activation of NST in man and animal species, which include the stimulatory effects of hyperphagia and, overnutrition during the early postweaning period, dietary macronutrient manipulation and environmental challenges on stimulating BAT growth and development.2–8 These studies demonstrated that early intervention may result in increased BAT growth via both hyperplasia and hypertrophy, and once formed, the greater BAT mass and cellularity may persist and remain active well into adulthood.7,8 The recent observations of Beige adipose tissue as an intermediate cellular type has also recently been reported by several authors, with the further suggestion that sympathetic stimulation may result in a transdifferentiation of Beige BAT into functional BAT adipocytes including the expression of the essential UCP-1 protein in the beige adipocytes.9 The interconversion of differentiated cell types from cells of fully differentiated tissues including BAT however may require a previously unknown epigenetic reversal were it to occur in fully terminally differentiated cells and would not only be a unique observation, but may be less convincing if it were to occur in older, more mature animals.
Because of the positive effects of BAT on thermogenesis, the inactivation of BAT-mediated thermoregulative processes via β-blockade, aging, thermoprotective environments, or thermal inactivation via rearing under environmental conditions that exceed the threshold for thermal neutrality would also be expected to reduce the thermogenic activity of brown adipocytes, thereby enabling the BAT tissues to increase intracellular lipid locule size and stored lipid content during periods when in a thermogenically less active state. Decreasing thermogenic activity likely would have the effect of a physical dilution of the normally deeper brown coloration of thermally active BAT tissues via increasing cellular lipid content while retaining their distinctive cellular multilocular lipid morphology and centrally located nucleus typical of brown adipocytes. In contrast, during lipid accretion and enlargement of white adipocytes the WAT cells typically retain their peripheral nucleus and a flattened outer ring of cytoplasm surrounding a single large lipid droplet which may undergo reversible expansion or contraction in lipid content in response to variations in nutritional factors. In the obese phenotype of the LA/Ntul//-cp and SHR/Ntul//-cp rats, where the BAT tissues also took on an intermediate beige density of brown coloration, the percent of BAT lipid content was found to well exceed that of thermogenically active BAT, exhibiting enlarged lipid locules and modestly larger adipocyte diameters but that retained their central nucleus and that were morphologically similar to those observed following chronic β-adrenergic blockade.10,11 In contrast, during acute 3-day periods of starvation, resting metabolic rates were observed to decrease, and the intensity of the brown adipose coloration of the Interscapular BAT depots were observed to increase in intensity as the tissue mobilized lipid for thermogenesis, thereby resulting in smaller locule diameters and enabling a concentration effect of the pigmented mitochondrial hemochromes.11,12 The changes in BAT coloration and lipid content during acute starvation were consistent with activation of BAT-mediated thermogenesis as a physiologic contributor to homeothermic thermoregulation during decreased nutritional and environmental distress and a normal physiologic response to states of over- and undernutrition and the genetic predisposition for obesity.10–14
The establishment of the hypothalamus as an important potential anatomic site of origination of the observed defective mechanisms of energy metabolism common to obese rodents has gained traction from studies by Tulp and Tso and others, where hypothalamic implants from 14 day gestational homozygous lean animals were implanted in the 3rd ventricle of young 4 week old Zucker rats that were homozygous for the fatty (-fa/-fa) trait, and monitored for further development of the obese stigmata.15–17 Upon reaching adulthood, the RMR, neurosympathetic and thyroidal endocrine parameters, lipid profiles and degree of adiposity of implanted rats were similar to those of their lean littermates, while the obese offspring that received only a sham hypothalamus injection devoid of transferred hypothalamic cells or tissue factors developed the obese stigmata and impaired thermogenic and endocrine parameters normally as predicted.15,16 The hypothalamus serves as the primary interface between the endocrine and nervous systems, where it facilitates the fine tuning of homeostasis for multiple diverse physiologic mechanisms including appetite and weight control, thirst and body fluid balance, emotion and sex drive, blood pressure regulation, thermoregulation, sleep cycles, growth development and maturation of mammalian organisms and others.17 As such, the functional integrity of the hypothalamic tissues is paramount to maintain homeostasis including the development of WAT and BAT tissues and their impact of elements of intermediary metabolism including mechanisms of thermogenesis and energy balance that may impact on adiposity and body composition. While the epigenetic locus of the (-fa) and (-cp) traits has been traced to chromosome number five by Liebel et al, the further intermediate biogenetic processes which enable the obesity traits to contribute to and to facilitate the expression and development of the obese+NIDDM stigmata remain unclear.18
One cardinal finding of the obese phenotype in obese man and animals is the development of the phenomena of insulin resistance (IR), especially as it occurs in skeletal muscle and adipose tissue, which represent the majority of the tissues where IR is expressed and which often exhibit the process of delayed glucose uptake in those tissues. Insulin resistance may occur via a number of mechanisms, but cellular glucose uptake in insulin-dependent tissues requires the presence of Glut4 transporters that facilitate the uptake of glucose at the plasma membrane of those insulin dependent tissues including skeletal muscle and adipose tissue.19 The intracellular translocation of GLUT4 transporters originating in the endoplasmic reticulum may be suppressed under conditions of dysregulated glucocorticoid actions often common in the obese state. The development of insulin resistance which may result from the disruptions in GLUT4 translocation may be a common occurrence in obese rodents where it may occur in both diabetic (NIDDM) and non-diabetic obese animals of either sex. However, the NIDDM stigmata are typically more pronounced in the males than the females of the various NIDDM-prone strains.20
The Wistar fatty Rat of this study, now officially established as WDF/Ta- fa x WKY/N crosses were originally designated as the WDF/TaDrt- fa strain by the Institute of Laboratory Animal Resources [ILAR], National Research Council of the NIH.20,21 The Wistar rat originated from an outbred colony of white rats of unknown origin that had been established at the Wistar Institute in 1906 and sold worldwide from the early 20th Century.22 The Wistar diabetic fatty (WDF/Ta- fa ) rats were later developed by Ikeda et al. at the Takeda Chemical Industries (Japan) by crossing the 13M Zucker ( fa/fa ) rat with the somewhat carbohydrate-intolerant lean Wistar Kyoto rat that had been established in their laboratory.21–24 The stated goal was to develop a rat model of non-insulin dependent diabetes mellitus (NIDDM) that would express metabolic characteristics including elements of adult onset and varying degrees of obesity, hyperglycemia, hyperinsulinemia, insulin resistance, glucose intolerance, and peripheral neuropathy that were similar to those which occur in human adult onset diabetes (NIDDM). A group of the diabetic fatty rats were then crossed with WKY/N rats maintained as an outbred colony by the Diabetes Research Training Center of the University of Indiana (DRTC) by Peterson et al to produce the current strain of virus antibody free (VAF) Wistar Fatty Rats of the current study.20 The epigenetic locus or loci that result in the expression of NIDDM stigmata are complex and remain unclear, but appear to be closely associated with the common trait leading to obesity as an autosomal recessive trait in the rat.20,21–24 Thus, the purpose of the present study was to further characterize the effect of the obesogenic -fa trait on parameters glycemic stat as performed in our laboratory for many years. us following glucose administration and non-shivering thermogenesis, two fundamental glucose dependent processes.
Groups of adult lean and obese Wistar Fatty Rats were obtained from the Diabetes Research Training Core Laboratories (DRTC), University of Indiana at 20 weeks of age, and placed in hanging steel cages by littermate pairs (1 lean +1 obese) with free access to Purina Chow #5012 and house water. Environmental conditions were adjusted to a reverse light cycle (dark 0800-2000 hours) maintained at 20±1°C and 50% relative humidity. At 22 weeks age the diet was switched to a semisynthetic diet recommended by Michaelis et al at the Carbohydrate Nutrition Research Laboratory of the USDA in Beltsville, Maryland, USA.25 The diet consisted of (w/w) 54% CHO as equal parts sucrose and cooked cornstarch, 16% mixed fats as equal parts lard, beef tallow, corn oil and coconut oil, 20% protein as equal parts casein and lactalbumin, 4% essential vitamins and minerals, and 5% cellulose from 22 to 30 weeks of age. Rats were weighed weekly to the nearest gram on an Ohaus animal balance. Measures of resting and norepinephrine stimulated thermogenesis were performed in rats after an overnight fast at 30 weeks of age via indirect calorimetry at thermal neutrality (30°C) and expressed as ml O2/kg BW-0.75 to correct for differences in body surface area between lean and obese rats as described by Kleiber and Yang et al as performed in our laboratories for many years.7,26–29 At 30 weeks of age rats were sacrificed by decapitation, truncal bloods collected, and principal fat depots excised in their entirety for quantification of measures of adipocyte size and number using the osmium tetroxide fixation technique for white adipose tissue and the double formalin-osmium fixation methods described by Tulp et al.6,8,28 The resulting fixed adipocytes were then analyzed on a Coulter particle counter equipped with a 400 micron aperture and a C-100 channelyzer and logarithmic expander unit (Coulter Electronics, Hialeah FL USA). Tissue lipid content was determined following lipid extraction of weighed sections with the method originally described by Hirsh and Gallian.29 Glucose was measured via a glucose oxidase method and insulin via solid phase radioimmunoassay. Data were analyzed via standard statistical procedures. The Institutional Animal Care and Use Committee approved the study.
The effects of phenotype on body weights and primary adipose tissue depots are presented in Table 1 and indicate that both the initial body weights taken at 22 weeks of age and the final body weights taken at 30 weeks of age were significantly greater in the obese than the lean phenotype. In addition, the weights of the dorsal epididymal, and retroperitoneal fat depots were also greater in the obese than the lean phenotype, with the most significant increase in the visceral retroperitoneal depots and which reflected a 15-fold increase over their lean littermates. When the combined weight of the 3 depots is expressed as a proportion of body weight, the obese rats again reflected a significantly greater proportion of adiposity than their lean littermates.
|
|
|
Grams per Depot |
|
|
|
|
Group |
N |
IBW |
FBW |
EPI |
DORSAL |
VRP |
ΣWAT: FBW |
Lean |
11 |
447±22 |
470±21 |
7.5 ±0.2 |
0.95±0.05 |
9.5 ± 0.4 |
0.038±0.002 |
Obese |
10 |
690±34 |
810±38 |
17.2±0.6 |
18.5±0,7 |
155.1±7.8 |
0.236±0.012 |
P = |
|
<0.01 |
<0.01 |
<0.05 |
<0.01 |
<0.01 |
<0.01 |
Table 1 Body weights and white adipose tissue characteristics of male lean and obese wistar fatty rats
Data are mean ±1 SEM, n=<11 rats/group. Initial body weights (IBW) at 22 weeks of age, final body weights (FBW) at 30 weeks of age. The sum of the 3 depots / FBW is reflected in the far-right column. EPT, epididymal fat pads; DORS, dorsal white fat pad; VRP, visceral retroperitoneal fat pads. All depots were excised in their entirety and promptly weighed to the nearest 0.05 g on a Mettler balance. Abdominal and other peripheral subcutaneous depots not determined.
The cellularity of the adipose depots is depicted in Figures 1 & 2 and indicate that the adipocyte lipid content was similar in the epididymal depots of both phenotypes, with only a modest increase in adipocyte number in the obese phenotype. There were significant increases in both cell lipid content and cell number per depot in the dorsal and visceral retroperitoneal depots. In those depots, the greatest increase in both adipocyte lipid content and adipocyte number occurred in the visceral retroperitoneal depot where the depot mass was significantly 15-fold greater than in their lean littermates, the cell numbers/depot were over 5.5-fold greater and the cell lipid content 2.5-fold greater than in their lean littermates. In that depot, the adipocyte lipid content approached the typical maximum of approximately 1.0 to 1.2 µg lipid content per adipocyte sometimes reported for adipocytes of white adipose tissue. Once formed, adipocytes of both types may be retained throughout much of the remaining lifespan, varying only in cellular lipid content once formed.
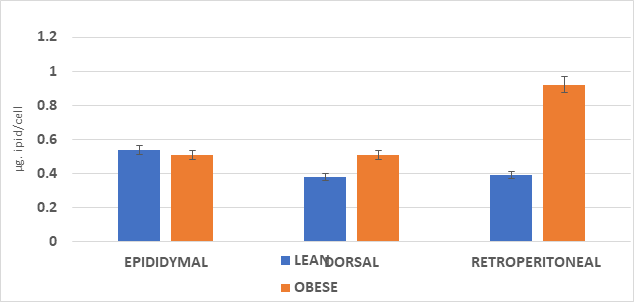
Figure 1 Adipocyte lipid content of lean and obese rats.
Data are mean ±1 SEM, n= <11 rats/group as µg lipid per cell. Abdominal and other peripheral subcutaneous depots not determined. Both epididymal pads and both visceral retroperitoneal depots dissected in their entirety. Cellularity measures determined on 50 mg representative aliquots of the respective tissue depots.
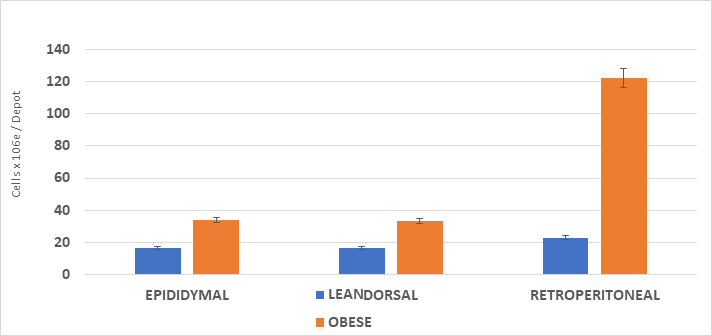
Figure 2 Adipose depot cell number in lean and obese rats.
Data are mean ±1 SEM, n= <11 rats/group cells x 10-6 / depot. Abdominal and other peripheral subcutaneous depots not determined. Both epididymal pads and both visceral retroperitoneal depots dissected in their entirety. Cellularity measures determined on 50 mg. representative aliquots of the respective tissue depots.
The mass and cellularity of the interscapular brown adipose tissue is depicted in Table 2 and indicates that the total mass of the IBAT depot of obese rats was significantly over 6-fold greater than was found in their lean littermates. When cellularity was determined, both cellular lipid content and IBAT cell number per depot were significantly greater in the obese than the lean phenotype with the most significant increase in the cell number per depot. This is indicative of hyperplasia of brown adipose tissue with a magnitude of increase that is reflective of early overnutrition in other rodent strains and similar to that which has been observed in other obese rat strains. Despite the significant increases in IBAT cellularity, measures of resting VO2 were decreased in the obese phenotype (Figure 3) and the increase in VO2 following a maximal dose of norepinephrine depicted in Figure 3 was less than half that observed in the lean phenotype (+33% in obese vs +79% in lean).
Group |
N |
G/Depot |
IBAT:BW |
Cells/Depot |
Cell Size, µg lipid/cell |
Lean |
11 |
0.624±0.067 |
1.3±0.1 |
4.62±1.18 |
O.399±.020 |
Obese |
10 |
4.265±0.367 |
5.3±0.3 |
12.19±3.64 |
0.499±0.079 |
P= |
± |
<0.01 |
<0.05 |
<0.01 |
<0.05 |
Table 2 IBAT parameters of male lean and obese wistar fatty rats
Data are mean ±1 SEM, n=8 rats/group. IBAT:BWx100; Cell number/depot expressed x 106 IBAT depots dissected free of visible white adipose tissue infiltrates.
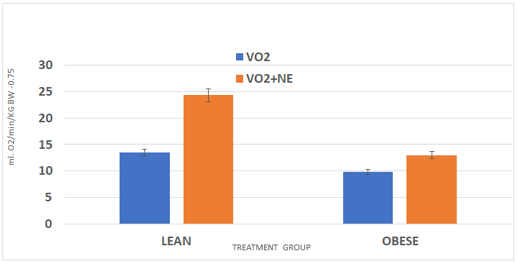
Figure 3 Resting and ne-stimulated vo2 in rats.
Data are mean ±1 SEM, n= 6 rats/group. P = < 0.01, VO2 vs VO2+NE in lean, p-<0.05 in Obese VO2 vs VO2+NE.
Measures of fasting glucose, Insulin, and the Insulin to glucose ratio, are depicted in Figure 4. These data indicate a normalization of fasting glucose concentrations in lean and obese rats, but a significant residual fasting hyperinsulinemia and significantly elevated Insulin to glucose ratio in the obese phenotype. The increased fasting insulin and the fasting Insulin to glucose ration is entirely consistent with insulin resistance in the obese phenotype. In addition, as reflected in the far-right panel of Figure 4, the adrenergic stimulated increase in fasting glucose concentrations following adrenergic stimulation was highly significant in the obese NIDDM phenotype and was more than twice the magnitude of the glycemic response observed in the lean littermates. The glycemic responses to NE administration are most consistent with hepatic glycogen mobilization in both phenotypes, with the greatest increase noted in the obese NIDDM phenotype.
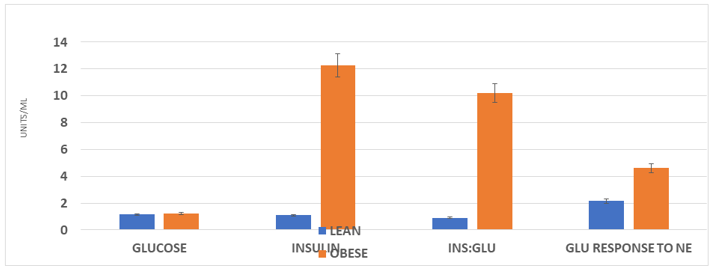
Figure 4 Effect of phenotype on glucose, insulin and glucose response to exogenous.
Data are mean ±1 SEM, N= 8 rats/group at 30 weeks of age. Glucose reported as mg/ml; Insulin as ng/ml, and insulin to glucose ratios as the arithmetic computation where insulin is the nominator and glucose the denominator. P = < 0.01 for Lean vs obese for insulin and insulin: glucose ratios. GLU response to NE fold-increase in blood glucose taken one hour after a maximal (200 µg/kg BW) NE subcutaneous injection.
The results of this study indicate that the capacity for activation and expression of non-shivering thermogenesis is impaired in the obese phenotype of this strain, despite abundant increases in the mass and cellularity of beige appearing brown adipose tissue in the interscapular depot. This decreased capacity for energy expenditure was associated with insulin resistance as evidenced by the abnormally elevated insulin to glucose ratio and accompanied with marked increases in adiposity as confirmed by increases in both adipocyte number and cell lipid content in principle white adipose tissue depots. Adipocytes from WAT are typically characterized by a single large lipid droplet which may often contain up to one microgram of lipid, surrounded by a thin outer layer of cytoplasm and exterior plasma membrane and a flattened appearing nucleus embedded in the cytoplasmic ring. In contrast, the beige and brown adipocytes are histologically of smaller overall diameter, and normally contain a more centrally located somewhat spherical nucleus, surrounded by multiple small lipid locules and abundant specialized mitochondria distributed throughout the cytoplasmic compartment of the cell. In addition, brown adipocytes can express the unique UCP-1 uncoupling protein found in BAT tissues and which is an essential element in the origination of the thermogenic cascade. When activated, the cascade can lead to the generation of heat energy that may then be efficiently dissipated by peripheral tissues via the strategically located vascular network surrounding the interscapular, thoracic and other BAT depots. Thus, BAT performs an essential role in maintaining thermal homeostasis during a variety of environmental and nutritional conditions including the arousal from states of torpor and hibernation in animals and in response to perturbations in dietary and environmental factors in both man and animals. The BAT development and maturation requires a number of hormonal stimuli including the lipogenic actions of insulin, and the release of myokine peptides including AKT1 and AKT2, which may play a role in the transition between BAT cellular types including both beige and brown cells. This maturation and differentiation can occur in the presence of hyperinsulinemia common to obesity and NIDDM and under conditions of dietary overnutrition and caloric imbalance.
The multiple BAT locules of brown adipocytes may contribute yet another important distinction between white and brown fat cells, as the many small lipid locules of brown and beige adipocytes may contribute a disproportionately large surface area to lipid volume. The greater surface area to volume ratio upon catecholaminergic stimulation can enable a more rapid surface mobilization and thermodynamically efficient release of the essential fatty acids contained and generate a more rapid response to diet and environmental conditions than would be likely in the single larger diameter lipid droplet of WAT cells. In WAT adipocytes, the lipid surface area relative to lipid volume is low in comparison to that of BAT cells owing to the differences in net available surface area from the multiple small locules vs the single large droplet. The relationship between surface area lipid volume becomes important as the lipid mobilization occurs at the lipid surface interface, and in this instance overall size and surface area of the available lipid deposits may makes a thermodynamically favorable difference in the capacity for the rates and magnitude of FFA mobilization under most physiologic conditions. The lipid content of both WAT and BAT depots is biochemically plastic in nature and can readily undergo expansion and contraction depending on conditions of positive or negative energy balance being experienced by the animal.
The BAT is the only tissue of homeotherms that has the primary function of heat generation in response to changes in diet and environment via nonshivering thermogenesis mechanisms, where it contributes to energy balance and regulation of homeothermy.2–5 Hyperinsulinemia common to obesity and NIDDM has been shown to impair thermogenesis in isolated brown adipocytes from obese and obese-NIDDM rats presumably at least in part via impaired cellular glucose uptake during the thermogenic process.30–32 The presence of insulin resistance in BAT and other peripheral tissues and the loss of insulin sensitivity in effecting glucose uptake in BAT tissues was likely a factor in the decreased expression of nonshivering thermogenesis observed in the present study. Thus, the mass and cellularity of BAT is not by itself a reliable marker of endogenous thermogenic activity, but may reflect dietary, environmental, genetic, and age-related influences in its development and thermogenic activity in this and other strains of obese and obese+NIDDM rats. This impairment is especially prominent in the presence of decreased insulin sensitivity that may impact on the efficiency of glucose uptake in BAT and other insulin dependent peripheral tissues. The BAT tissues are innervated by individual noradrenergic sympathetic neurons which release norepinephrine which can impinge on stereoselective β3 receptors that are unique to brown adipose tissues. The neurohormone may therefore demonstrate comparable thermogenic responses following both the endogenously released and exogenously administered norepinephrine. Subcutaneous administration especially in the upper dorsal region in close proximity to the interscapular depot typically results in peak responses within 15 minutes and which are sustained for 20 minutes or more, dependent on the dosage administered. In the present study a dosage of 200 µg/kg body weight was administered, which was associated with a peak thermic response within minutes after administration and which typically persisted for 15 minutes or more in both phenotypes.33
Catecholamines can bring about the adrenergic mobilization of glucose from available glycogen stores, including those contained in hepatic and muscle tissues. The bulk of the glucose mobilized from skeletal muscle is dedicated to intracellular use, where it can provide substrate for muscular activity. In contrast, the bulk of the glucose released from glycogen mobilized from hepatic storage is destined for the peripheral circulation, including the CNS and other tissues, where glucose uptake may occur independently of GLUT4 transporter activity.34,35 In the present study, the NE resulted in significantly greater glucose concentrations in the obese than the lean phenotype an hour after the NE challenge. The greater response is likely a combination of the greater hepatic glycogen stores commonly associated with NIDDM and an impaired peripheral uptake in insulin dependent tissues including skeletal muscle, where much of the insulin dependent peripheral glucose uptake occurs.
Glycogen degradation is well established to play an important role in the regulation of blood glucose concentrations, where it is important in maintaining glucose availability for substrate oxidation in neural, muscular and other peripheral tissues under a broad spectrum of physiologic conditions.32–34 While the biochemical mechanisms and the hormonal and metabolic signals for glycogen biosynthesis are similar in both muscle and hepatic tissues, the glycogen stores from both sources are uniquely adapted to the specific roles of glycogen mobilization in their respective tissues. In myocytes, both hormonal and neural signals can initiate glycogen degradation to form glucose-6-phosphate as the predominating end-product, which is impervious to membrane translocation but can efficiently enter glycolysis and high energy phosphate formation (i.e., ATP) in the cytosolic and mitochondrial compartments of the myocytes.4,34 Because glucose-6-phosphate can be readily oxidized in myocytes via glycolysis, it can thereby provide an immediate energy source during muscle exertion and fight or flight situations. Thus, in muscle tissues, most of the glucose moieties generated are reserved primarily for intracellular use due to an absence of the glucose-6 phosphatase activity, needed to dephosphorylate the glucose-6-phosphate to form free glucose and thereby not enable the phosphorylated glucose moieties to exit the myocytes. In contrast, hepatic tissue has an active glucose-6-phosphatase enzyme and can generate free glucose for extrahepatic use. Accordingly, glucose generated following glycogen mobilization can readily exit hepatic tissues via facilitated diffusion channels with the assistance of a GLUT2 transporter mechanism, thereby providing free glucose for uptake and oxidation in all extrahepatic tissues. In the present study, administration of the neurohormone norepinephrine resulted in significant increases in blood glucose concentrations which were of greater magnitude in the obese than their lean littermates one hour after the neurohormone was administered. The greater glycemic response to norepinephrine in the obese-NIDDM phenotype is likely due to a greater glycogen availability in the livers of the obese-NIDDM animals, combined with delayed post-administration uptake in insulin dependent tissues including muscle and adipose tissues. The delayed glucose uptake likely occurred as a consequence of insulin resistance in those tissues and organs. Measures of Glucose tolerance in the obese-NIDDM WFRs also indicated a delayed glucose uptake among the obese phenotype of this strain.32–34
The increased mass and cellularity in both BAT and WAT depots in the obese-NIDDM phenotype was anticipated as a logical consequence to chronic overnutrition and is consistent with overfeeding-induced hyperplasia and hypertrophy in those tissues.34 Once formed, the adipocytes in both BAT and WAT depots likely remain present well into adulthood, where they may continue to perform their metabolic functions including energy expenditure and storage respectively. In other studies, the hyperplasia in BAT depots appeared to continue until approximately adolescence in the rat and can increase by hypertrophy thereafter resulting in the physiological graded ‘unbrowning’ phenomena of brown adipocytes toward a beige coloration as the lipid locules increase in size and brown adipocyte lipid content becomes progressively increased and the mitochondrial and hemochromic pigments become less densely located following the excess lipid accumulation. Pharmacologic inactivation of brown adipose tissue via β-adrenergic blockage can also bring about an increase in dimensions of the lipid locules and increase tissue lipid content in a manner similar to that which occurred in the apparent ‘unbrowning’ in the obese+NIDDM rats.10-14 Whether a full restoration of the thermogenic activity of BAT from obese+NIDDM rats in response to environmental or nutritional factors would become more completely effective is unclear, as the tissue can still generate the essential thermogenic UCP-1 protein, but the coexistence of decreased insulin sensitivity has been demonstrated to impair the thermogenic responses in isolated brown adipocytes and in intact animals. In addition, a compromise in other insulin-linked metabolic functions including the rate and efficiency of protein turnover in muscle and other peripheral tissues has been reported.26,29 The obese of this and other obese rodent strains have been observed to enjoy an improved caloric efficiency of energy deposition with advancing age when compared to their lean littermates, a secondary effect of insulin resistance and which contributes to the progressive development of the adiposity resulting in the obese and obese+NIDDM states.28,29,31–35 Although the peripheral abdominal and other subcutaneous depots were not dissected or analyzed in this study, an early study in Sprague Dawley rats demonstrated progressive increases in adipocyte size and number could occur well into adulthood, and likely function as a suitable energy reserve for energy deposition particularly in response to states of chronic overnutrition.28 The thermogenic responses observed in BAT depots occur via a combination of direct sympathetic innervation and thyroidal actions, as BAT tissues are an active site of T4 5’ deiodinase activity, where they facilitate the enzymatic intracellular conversion of T4 to its hormonally and metabolically active form, in close proximity of the T3 nuclear receptors, in addition to having stereospecific β3- adrenoreceptors strategically located on the plasma membranes of brown adipocytes.36 Thus, the activation of BAT-mediated thermogenesis is normally a complex multilayered but intimately coordinated process that can rapidly adapt to acute and chronic changes in dietary and environmental stimuli, with corresponding variations in the capacity for maintaining homeostasis and energy balance, but which may undergo dysregulation in the presence of decreased insulin sensitivity, insulin resistance, obesity and NIDDM.
The capacity for expression of parameters of non-shivering thermogenesis has been demonstrated to decreased in several animal models of genetic obesity, where the obese trait is expressed in a variety of different background strains that vary in their metabolic characteristics and their propensity to develop pathophysiologic sequelae. In summary, the present study employed a strain derived from a cross between a diabetogenic Wistar-Kyoto rat and incorporating the obese trait from a Zucker fatty rat. The consequence resulted in offspring that demonstrate overt characteristics of obesity+NIDDM in one quarter of the offspring of heterozygous breeding pairs, and where the obesity+NIDDM occurs in both genders of the offspring by mid to late adolescence. In the current study, male obese+NIDDM rats demonstrated an impaired capacity for nonshivering thermogenesis association with significant increases in both adipocyte lipid content and adipose cellularity and in relative adiposity in the obese+NIDDM phenotype of the Wistar Fatty Rat strain. The obese phenotype of the WFR strain demonstrated measurable increases in the fasting Insulin to Glucose ratios, consistent with longstanding insulin resistance, an established factor in the impaired BAT-mediated thermogenesis in isolated brown adipocytes in vitro. Both lean and obese rats demonstrated a response to exogenous norepinephrine administration, although the thermogenic response of the lean phenotype was approximately twice the magnitude of the lean phenotype. The dose of the NE administered was greater than than was used in other studies with obese rats, which likely contributed to the greater response. A unique observation of this study was the measurement of the glycemic increase following the NE administration, which showed that the glycemic response of the obese rats was more than twice the magnitude of the lean rats. This is likely significant, as the plasma glucose can now enter many extrahepatic tissues in addition to BAT depots, all of which can contribute to the net thermogenic responses as likely cascade effects of glycolytic activity in those tissues, even in the presence of insulin resistance. The measures of resting and norepinephrine stimulated thermogenesis were performed in quietly resting fasted animals, where the overnight fast may have enabled a limited upregulation of insulin receptors as the T1/2 for circulating insulin and its receptors differ sufficiently and may enable a partial restoration of glucose uptake processes in myocytes and adipocytes, thereby contributing to the thermogenic response to norepinephrine. The glycemic response to norepinephrine is a unique finding in the study, not previously reported in other rodent strains of obesity.
Glycogen storage in hepatic tissues is typically greater in NIDDM than occurs in non-NIDDM or IDDM states, likely due to the differences in circulating insulin availability in the two forms of diabetes. Insulin is not an absolute requirement for glucose uptake in hepatic tissues, while the prolonged increases in circulating glucose concentrations in NIDDM can provide viable substrate for glycogen deposition. In applications to ports nutrition, a similar technique is sometimes employed in the process of glycogen loading, which has been shown to bring about increases in both skeletal muscle and hepatic glycogen content, and which has been proposed as one of several factors that may enhance aerobic athletic endurance and performance in competitions such as running, skiing and track events. Glycogen may be mobilized following hormonal actions of glucagon in fasting, and catecholamines following endogenous release or exogenous administration. In the present study, exogenous administration of norepinephrine was followed by a profound increase in plasma glucose concentrations in both phenotypes, with the greatest response in the obese+NIDDM phenotype, although it could not be determined if the greater glycemic response in the obese+NIDDM phenotype occurred as a consequence of a quantitatively greater glycogenolytic response, a delayed peripheral glucose uptake, or some combination of the two factors which is highly likely due to the magnitude of insulin resistance observed.
The effect of early hyperphagia following the feeding of highly palatable cafeteria (Café) diets to normally lean rats during early postweaning growth (postweaning days 21 to 42) resulted in increases in plasma T3, RMR, and in hyperplasia of the Interscapular brown adipose depots and the feeding of unbalanced, protein deficient but calorically adequate diets resulted in similar findings, although other parameters of linear and physical growth were impaired.6,8 Thus, early hyperphagia in the early postweaning period would appear to trigger hyperplasia in BAT is an apparent physiologic mechanism to dispense the excess caloric intake during the postweaning period, and at a time in postweaning development when pre-brown adipocyte may still undergo replication and resulting in an increased BAT mass. While in genetically lean rats, in the absence of obesity or obesity+NIDDM stigmata, including progressive increases in generalized insulin resistance, an established factor in impaired thermogenesis in isolated brown adipocytes.7,27,31–35 Regardless of the contributions of the various parameters of energy balance and thermal homeostasis, the obese phenotype of this strain demonstrated significant impairments in parameters of non-shivering thermogenesis, and which likely contributed to the progression of the stigmata of obesity and NIDDM. These stigmata included hyperplasia and hypertrophy of white adipose tissue depots as well, with the greatest increases noted in the visceral abdominal retroperitoneal depots of those sites measured in this study. In humans, increases in visceral abdominal fat depots tend to carry a greater risk for the development and progression of cardiovascular disorders and other comorbidities, but such differences in body fat distribution patterns have not yet been fully reported in this or other obese rat strains, most of which have a lower apparent predisposition for cardiovascular pathophysiology, and were not assessed in the current study.
Obesity and NIDDM and their common sequelae typically become chronic comorbidities in Western Society, and their prevalence is increasing at an alarming rate despite considerable new knowledge in areas of clinical nutrition and pharmacotherapies that may be useful in attenuation of the symptoms of the disorders.37,38 Once diagnosed, obesity and NIDDM require frequent and continuing clinical attention, and have now placed a significant strain on medical resources in many countries. The losses include not only a financial burden on the individuals and the medical resources consumed in their patient care, but also contribute to creating an additional burden on the global and regional economies due to the losses incurred due to decreased productivity of affected workers. Thus, the application of useful animal models to explore new avenues of diagnosis and treatment can add innovative new approaches to the management of obesity, NIDDM and their comorbidities, with a long-term goal toward the betterment of man and society and a healthier tomorrow.
None.
The authors declare that they have no conflicts of interest.
None.

©2022 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.