Advances in
eISSN: 2378-3168


Mini Review Volume 12 Issue 3
1NorthamptonGeneral hospital, University of South Wales, UK
2Al-Neelain University, Sudan
3Northampton General Hospital, UK
Correspondence: Tahir Omer, Northampton General hospital, University of South Wales
Received: May 21, 2022 | Published: June 2, 2022
Citation: Omer T, Suareldahab I, Hamid RGA. Patient selection for bariatric surgery: a review of health outcomes, safety and the role of the MDT. Adv Obes Weight Manag Control. 2022;12(3):71-76. DOI: 10.15406/aowmc.2022.12.00366
The pandemic of Obesity with its rapidly rising prevalence and burden on the health system, has become a major health concern. A variety of modifications including dietary, physical activity changes, behavioural in addition to pharmacotherapy interventions have been recommended for obesity management. Bariatric surgery has also been recommended for individuals with a Body Mass Index (BMI) exceeding or equal to 40 kg/m2 or in the range between 35 kg/m2 and 40 kg/m2 with a simultaneous significant comorbidity.
Health practitioners often face the dilemma of patient selection when making a decision on bariatric surgical procedure suitability. This selection process is key to attaining good surgical outcome. Therefore, the input of a profecient multi- disciplinary team is crucial to the process. The following brief review is to point out the factors that influence the selection process. It describes the diverse bariatric surgical procedures available, discusses their efficacy, safety and complications rate, and emphasizes on the role of the MDT team in procedure selection according to patient characteristics.
Keywords: obesity, bariatric surgery weight loss, patient selection, obesity MDT
Obesity, with its rapidly rising prevalence and burden on the health system, has become a major health concer.1,2 A variety of modifications including dietary, lifestyle changes, physical activity, behavioural in addition to pharmacotherapy interventions have been recommended by the national institute for health and care excellence (NICE).2–4 In the event of failure of the above mentioned methods to achieve beneficial weight loss, surgical interventions commonly known as bariatric surgery is recommended for individuals with a Body Mass Index (BMI) exceeding or equal to 40 kg/m2 or in the range between 35 kg/m2 and 40 kg/m2 with a simultaneous significant comorbidity.
The National institute of diabetes and digestive and kidney disease has approved the use of bariatric surgical techniques for those with a BMI of 30 kg/m2 or more with a difficult to control type 2 Diabetes concurrently.5
Bariatric surgery is a renowned and prominent treatment option for obesity that is growing in popularity. Statistics have shown a significant increase in the number of bariatric procedures performed in 2018 from nearly 228,000 in 2017 up to 252,000 as stated by the American Society for Metabolic and Bariatric surgery (ASMBS).6
The following brief review is to point out the diverse bariatric surgical procedures available, discuss their efficacy and safety and emphasize on the role of the MDT team in procedure selection according to patient characteristics.
Historical bariatric surgical procedures
Earlier attempts to address obesity through surgical intervention included Jaw wiring which was proven ineffective due to the inability to sustain control on the consumption of calorie-rich liquid diet.6
Jejunoileal bypass was also considered historically. This involved excluding large part of the intestine and had a high rate of serious complications including nutritional and electrolyte deficiencies, hepatic and renal complications. This subsequently led to abandonment of the procedure.
Gastric partitioning with a stapling device and vertical banded gastroplasty (VBG)is another historical procedure with high failure rate due to recurrent disruption of the staples or disappointing weight loss outcome.6
Present bariatric surgical procedures
Bariatric surgery procedures can be broadly classified into 3 categories: restrictive, malabsorptive, or a combination of the two.
Laparoscopic adjustable gastric banding (LAGB)
This involves positioning a band to constitute a small pouch of the stomach just underneath the gastroesophageal junction.7 In 2009, more than 56% of bariatric surgical procedures worldwide were LAGB.8 LAGB is categorised as a restrictive procedure. However, the created small pouch of stomach seems unable to even tolerate a small amount of food. Thus, the mechanism of action has been theorised to also include induction of satiety.9
Once considered the gold standard in bariatrics, the use of LAGB has declined recently due to emerging unsatisfactory complication rate.7,9,10
Sleeve gastrectomy (SG)
This involves excluding a large part of the stomach reducing it to about 15% of its original size by surgically removing a large portion along the greater curvature. It is minimally invasive and less technically challenging than other procedures.10 SG falls under the criteria of all the modalities classification (restrictive, malabsorptive and metabolic). It can lead to complex metabolic and endocrinological long-term effect. The reduction in the size of the stomach limits its ability to distend. Furthermore, it leads to a reduction in ghrelin secretion from the parietal cells which in turn reduces the hunger effect of the hormone. Alteration of the gut microbia takes place leading to significant appetite suppression. It also leads to higher levels of postprandial GLP1 and Peptide YY which delay gastric emptying.10
Roux-en-Y gastric bypass (RYGB)
This is usually done laparoscopically. It involves reducing the size of the stomach to a small pouch about the size of an egg. A Gastric pouch is created by transecting the stomach then the pouch is directly attached to the intestine creating a Roux-en-Y gastrojejunostomy, thus diverting nutrients from the stomach, duodenum, and proximal jejunum.11
Biliopancreatic diversion with duodenal switch (DS)
This is a malabsorptive complex procedure that includes sleeve gastrectomy. An anastomosis is created that bypasses the intestine leading to a significant degree of malabsorption of nutrients. It is associated with a higher incidence of complications and hence rarely performed.12
Weight loss
Table 1 & Figure 1 summarise some of the studies that put in comparison the effect of these bariatric procedures on weight loss.12 – 21 Most of the studies utilise the percentage of excess weight loss calculated as follows: [(operative weight – follow-up weight) / (operative weight – ideal weight)] x100.13–29
Study |
Participants |
LAGB |
SG |
RYGB |
DS |
Angrisani et al.15 |
51 patients (24 RYGB + 27 LAGB) |
%EWL 34.7% |
- |
%EWL 51.3% |
- |
Nguyen et al.16 |
250 patients (111 RYGB + 86 LAGB) |
%EWL 36.5% |
- |
%EWL 64.3 P < .05 |
- |
Himpens |
80 patients (40 SG + 40 LAGB) |
%EWL 41.1% |
%EWL 57.7%P < .001 |
- |
- |
Karamanakos et al.17 |
32 patients (16 SG + 16 RYGB) |
- |
%EWL 69.7 ± 14.6% |
%EWL 60.5 ± 10.7%. P = .41 |
- |
Kehagias et al.18 |
60 patients (30 SG + 30 RYGB) |
- |
%EWL 72.9% |
%EWL 65.6P = .05 |
- |
Zhang et al.19 |
64 patients (32 SG+ 32 RYGB) |
- |
% EWL 73.9% |
%EWL 84.5% P > .05 |
- |
Salminen et al.20 |
240 patients (121 SG + 119 RYGB) |
- |
%EWL 49% |
%EWL 49 |
- |
Peterli et al.,21 (SM-BOSS) trial |
- |
%EWL 61.1% |
%EWL 68.3% |
- |
|
Skogar and Sundbom22 |
113 DS and 98 RYGB |
- |
- |
% EWL 62% |
%EWL 79% P < .01 |
Hedberg, Sundstrom & Sundbom23 |
Systematic review of 16 studies including 874 DS and 1,149 RYGB |
- |
- |
- |
6.2 (95% confidence interval 5.0-7.5) BMI additional weight loss compared with RYGB P < 0.001 |
Table 1 A summary of the some of the studies that compared the efficacy of variable bariatric procedures on weight loss
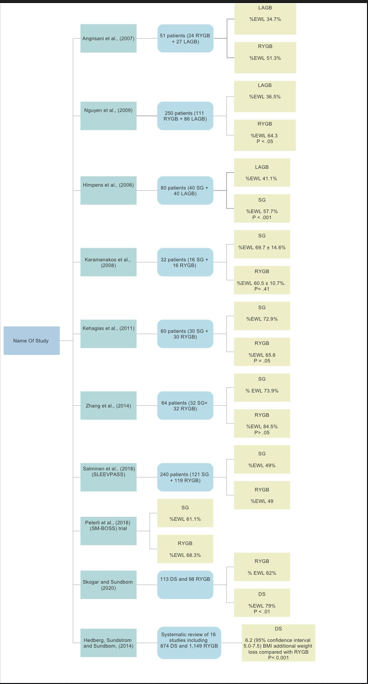
Figure 1 A summary of the some of the studies that compared the efficacy of variable bariatric procedures on weight loss.
It is evident from these studies that RYGB leads to a greater weight loss than LAGB. SG is also superior to LAGB in weight control. The Swedish Obese Subject SOS study22 demonstrated that at the 10-year follow-up, weight loss was 25% ± 11% of total body weight for RYGB and 13.2% ± 13% for GB in comparison to controls (1% ± 6% weight gain). LAGB is associated with slow weight loss over 3 to 4 years in comparison to the fast weight loss (over 1 to 2 years) associated with RYGB. DS is associated with greater weight loss than RYGB but might be limited by the higher rate of complications (Figure 2).
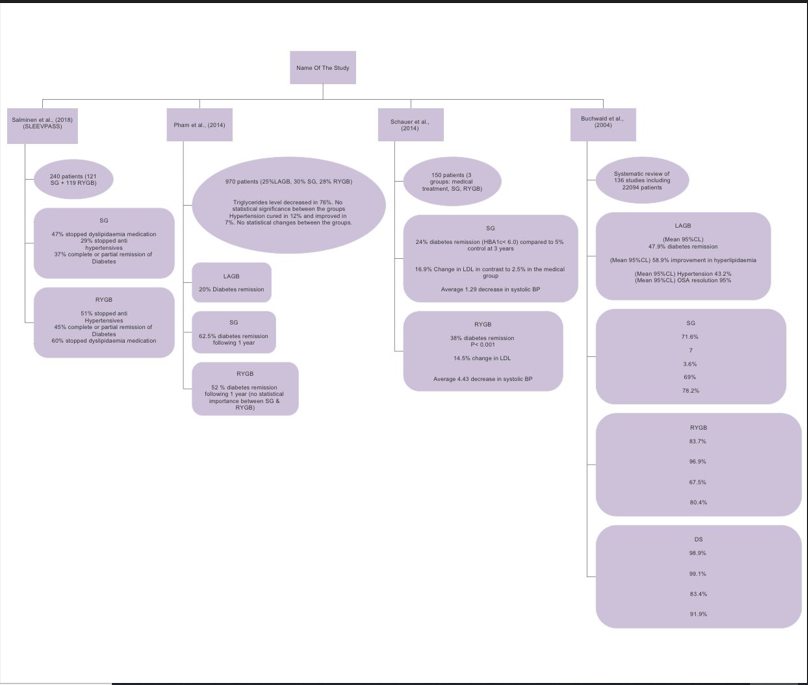
Figure 2 A summary of some of the important studies that compared the effect of different bariatric procedures on Diabetes and medical co-morbidities.
Type 2 diabetes remission, metabolic parameters and medical co-morbidities
Evidence has proved that Bariatric surgery can lead to improvement in type 2 Diabetes across all procedural types. It can also lead to improvement in different metabolic parameters including blood pressure, lipid metabolism and obstructive sleep apnoea. The Swedish Obese Subject SOS study22 demonstrated 2- and 10-year high rates of diabetes remission. Improvement in hypertriglyceridemia, levels of HDL, hypertension, and hyperuricemia were more favourable in the bariatric group than in the control. It has also shown 80% decrease in annual mortality in the surgical group. In addition, bariatric surgery leads to improvement of anxiety and emotional well-being through improving body image issues (Table 2).
Study |
Participants |
LAGB |
SG |
RYGB |
DS |
Salminen et al.20 |
240 patients (121 SG + 119 RYGB) |
- |
37% complete or partial remission of Diabetes |
45% complete or partial remission of Diabetes |
- |
47% discontinued dyslipidaemia medication |
60% discontinued dyslipidaemia medication |
||||
29% discontinued anti |
51% discontinued anti |
||||
hypertensives |
Hypertensives |
||||
Pham et al.25 |
970 patients (25%LAGB, 30% SG, 28% RYGB) |
20% Diabetes remission |
62.5% diabetes remission after 1 year |
52 % diabetes remission after 1 year (no statistical significance between SG & RYGB) |
- |
Hypertension resolved in 12% and improved in 7%. No statistical difference between the groups. |
|||||
Triglycerides level fell in 76%. No statistical difference between the groups |
|||||
Schauer et al.26 |
150 patients (3 groups: medical treatment, SG, RYGB) |
- |
24% diabetes remission (HBA1c< 6.0) compared to 5% control at 3 years |
38% diabetes remission P < 0.001 |
- |
16.9% Change in LDL compared to 2.5% in the medical group |
|||||
Average 1.29 drop in systolic BP |
|||||
14.5% change in LDL |
|||||
Average 4.43 drop in systolic BP |
|||||
Buchwald et al.27 |
Systematic review of 136 studies including 22094 patients |
(Mean 95%CL) 47.9% diabetes remission |
71.60% |
83.70% |
98.90% |
7 |
|||||
(Mean 95%CL) 58.9% improvement in hyperlipidaemia |
3.60% |
96.90% |
99.10% |
||
(Mean 95%CL) Hypertension 43.2% |
69% |
67.50% |
83.40% |
||
|
|
(Mean 95%CL) OSA resolution 95% |
78.20% |
80.40% |
91.90% |
Safety and complications
We have summarised the mortality and potential complications rate for each procedure in Figure 3.
Procedure selection and the role of the MDT
If the patient meets the NIH criteria for bariatric surgery, a full-scale thorough assessment of the suitability of the candidate is completed by the MDT team. This is an intricate process that involves psychological, surgical, nutritional and medical evaluation. The decision and choice of surgery takes into account the benefit to the candidate and the peri and post-operative risks. This should be individualized and should include managing expectations and assessing the patient commitment.11 Currently, there is no clear guidance on procedure selection. The choice of the procedure is determined by the individuals' objectives and peri-operative risk.28
The anticipated weight loss with each procedure should be contemplated. Although RYGB can result in greater weight reduction, close postoperative monitoring can enhance the weight reduction post LAGB. LAGB has a lower rate of early complications but a, higher rate of revision. RYGB has a higher mortality rate than LAGB yet remains < 0.5%. Momentarily, weight loss is comparable between RYGB and SG. However, in the long-term, more patients will regain weight post SG. DS is associated with the best weight reduction, but with highest rate of complication. It might not be appropriate for high-risk patients.
RYGB provides a greater chance of Diabetes remission than LAGB. SG and RYGB have a similar Diabetes remission rate, but SG has a higher rate of relapse. DS offers the highest rate of diabetes remission.
Obstructive sleep apnoea (OSA) and asthma could hypothetically be considered for more reliably efficient procedures such as DS or RYGB. Comparably, SG and LAGB can lead to deterioration in gastro-oesophageal reflux disease and, hence, should be avoided in this group.
In summary, candidate assessment and selection are crucial to achieve optimal surgical outcome. The selection of procedure is an intricate process taking into consideration the patients, their objectives and potential peri-operative risk. The surgeon's familiarity with the procedure type and its complications remains a prevailing contemplation. An up-to-date agreed consensus on patient selection and procedure to patient matching would be very helpful. However, individualisation of any treatment plan according to efficacy and safety is of a paramount importance.
None.
None.
None.

©2022 Omer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.