Advances in
eISSN: 2378-3168


Research Article Volume 9 Issue 1
1Kulvinder Kaur Centre For Human Reproduction, India
2Scientific Director Ex-Rotunda-A Centre for Human reproduction Consultant Neurologist, India
3Swami Satyan and Hospital, Near Nawi Kachehri, Baradri, Ladowali road, India
Correspondence: Kulvinder Kaur, Scientific Director, Centre For Human Reproduction, 721, G.T.B. Nagar Jalandhar-144001, Punjab, India, Tel 91-181-9501358180
Received: February 13, 2019 | Published: February 28, 2019
Citation: Kaur KK, Allahbadia Gs, Singh M. Have Probiotics and Synbiotics passed the test of time to be implemented in management of obesity and related metabolic disorders-a comprehensive review. Adv Obes Weight Manag Control. 2019;9(1):21-28. DOI: 10.15406/aowmc.2019.09.00269
With obesity growing by leaps and bounds ,being responsible for millions of deaths either directly or indirectly through its complications like type 2 diabetes mellitus (T2M), Metabolic syndrome, various cancers there is need for medical answers to effectively combat it.With the pharmaceutical drugs getting developed and shunted out of market with undesirable side effects directions are being shifted towards more physiological methods using some plant extracts like thylakoids, various anthrocyanins etc to combat Insulin Syndrome ( IRS),T2DM similarly attention is being moved to modify gut microbiota with the use of probiotics and synbiotics. Till date most beneficial strains have been developed from Lactobacillus or Bifidobacteria. Although a promising strategy both in animal and human models still human trials have remained few with inconsistent results. Hence need for long term studies with more bacterial strains are desired to ensure Probiotics become an established method of treating obesity .Thus we carried out this review to understand what is the status of use of these Probiotics and synbiotics in human trials in patients having obesity ,T2DM, Hypertension, IRS, nonalcoholic fatty liver disease (NAFLD)and steatohepatitis.
Keywords: obesity, T2DM, NAFLD, Cancer, Probiotics, synbiotics, bariatric surgery, medical pharmacotherapy of obesity
Obesity continues to be a big public health problem, with its prevalence increasing continuously. As per the WHO it has been estimate that In last 40 years, obesity prevalence almost tripled and in 2016, over 650million people around the world, which included various million infants and children became obese.1 Increased body weight is associated with development of several severe chronic conditions like type 2 diabetes mellitus, (T2DM), cardiovascular disease (CVD), musculoskeletal disorders and different cancers.2 Every year because of overweight/obesity, there are 28million deaths worldwide.2 Further obesity leads to a big medical, social and economic burden.3 We have been trying to find simple answers for treating obesity, medically, the problem remains that gradually most of the previous approved medications for obesity have got removed from the market, in view of different side effects, along with their inability to maintain long term weight loss.4–8 Although interventions like bariatric surgery are the most effective till date for reducing increased weight in people with morbid obesity, it is a very invasive procedure, having risks of unforeseen complications along with needing marked effort in adopting a new lifestyle [reviewed in ref.9 Thus need for looking simpler approaches is there. Symbiosis has been described in nonalcoholic steatohepatitis (NASH),10,11 T2DM, metabolic syndrome,12–15 and obesity,16,17 As far as overweight/obesity, is concerned various studies have demonstrated that the gut micro bio decomposition may be significantly different from lean individuals, the faecal bacteria may exert a key role in modulating energy metabolism with modifications of gut microbiotacomposition might be associated with decreases In body mass index (BMI)16,17 Reviewed in ref. 5,18,19 In view of this manipulation of gut microbiota composition using probiotics has been considered a possible way for preventing and treating obesity. The word probiotic comes from the Greek word, which means ‘’for life’. Despite ’lot of change in definitions, currently the definition recognized by Food and Agricultural Organization of the United Nations (FAO) and world health organization (WHO) working group experts is that probiotics are live strains of strictly selected microorganisms, which once administered in adequate amounts, give a health benefit to the host.20 This definition was accepted by the International Scientific Association of Probiotics and Probiotics (ISAPP) in 2013.21 Though dead bacteria and their components can also show probiotic properties. Most commonly used bacterial strains are Bifidobacteria and Lactobacillus that exhibit probiotic properties and get included in many functional foods and dietary supplements.22 Main mechanisms of actions of probiotics are improvement of the gut barrier function ,increasing competitive adherence to the mucosa and epithelium, modification of gut microbiota, along with regulation of the gut associated lymphoid immune system .Thus probiotics communicate with the host utilizing intestinal cell pattern recognition receptors ,like toll like receptors and nucleotide binding oligomerization domain containing protein like receptors that modulate important vital signaling pathways like nuclear factor kappa B (NFκB) and mitogen activated protein kinase to increase or suppress either activation or effect downstream pathways.21,23–26 A Probiotics is a nonviable food component which gives a health benefit on the host associated with modulation of microbiota, which might be a fiber, but all fibers are not necessarily a Probiotics. Usage of probiotic and Probiotics together is often known as synbiotic, if the net health benefit is synergistic27 Probiotics and synbiotics are taken in multiple and varied forms, like yogurt and other fermented milks, cheese and various fermented foods besides in the prevention and treatment of different gastrointestinal (GI) tract dysfunctions and other diseases like allergy.28 However the actual effects of how probiotic changes intestinal ecology is still debatable, in view of various confounding elements, like dissimilarities in microbial strains, concentrations of viable cells and product formulations.29–31 Yao and Kim showed that probiotics and Probiotics affect type2 diabetes mellitus (T2DM), and cardio vascular disease (CVD) by changing gut microbiota, regulating insulin signaling, along with lowering cholesterol.32 Thus the aim of this review was to study if probiotics and synbiotics are effective in the prevention along with treatment of obesity, IRS, T2DM, non alcoholic fatty liver disease (NAFLD) in human studies.
We carried out a Pubmed Search for articles related to obesity, Role of Probiotics in Obesity, type2 diabetes mellitus, nonalcoholic liver fatty disease/steatohepatitis, treatment with probiotics, Synbiotics from 1990’s to 2018.
We found a total of 863 articles relevant to this field of which we selected 76 articles for this review. Further references were obtained from cross references obtained from the original articles. No meta-analysis was done. On testing Lactobacillus salivarius Ls 33 in obese adolescents, for studying what effects it causes on fecal microbes, along with anthropometric data ,inflammation related biomarkers, carbohydrate and lipid metabolism, the following results were found. Ratios of Bacteroides, Prevotellae and Porphyromonas group of bacteria to Firmicutes-group ,that included Clostridium cluster XIV, Blautia coccoides, Eubacterium rectal group and Roseburia intestinalis were makedly increased following Lactobacillus salivarius Ls.33 But the overall cell numbers of fecal bacteria which included the above groups along with Clostridium cluster I and cluster IV, Faecalibacterium prausuitzi, Enterobacteriaceae, Enterococcus, Lactobacillus group and Bifidobacterium spp, were not changed much with this treatment. Also short chain amino acids (SCFA’s) remained unchanged.33 Further an intervention study was done by Gobel et al.,34 using Lactobacillus salivations Ls 33on effects of inflammation biomarkers along with different aspects of metabolic syndrome in adolescents having obesity. No changes were found in these parameters. Two studies used a cohort of Japanese adults having large visceral fat areas (VFA) to examine the effect of L. gasseri SBT2055.They assigned participants into 3 groups getting increasing colony forming units (CFUs) of L. gasseri SBT2055 for 12 weeks .A decrease in body mass index (BMI), waist, abdominal VFA and hip circumferences were noted[35,36].Similarly Sharafeditinov et al studied use of a hypocalorie diet that was supplemented with a probiotic enriched cheese that contained Lactobacillus plantarius and found this decreased the BMI, the putrescine content and the intestinal lactobacillus content in Russian adults having obesity along with hypertension. A lower diastolic blood pressure along with tendency towards lower systolic BP was seen in this group.37 Giving Lactobacillus. Acidophilus La 5, B. lactis Bb 12 and L. Casei was examined in people having high BMI, after randomly dividing them into 3 groups based on a specific intervening diet; first group received regular yogurt with low calorie diet (RLCD). The second one got a Probiotics yogurt with low calorie diet (PLCD),While 3rd received a probiotic yogurt without low calorie diet (PWLCD) for roughly 2months.A decrease in BMI, fat percentage and leptin was seen and was more so in groups that received weight loss diet including probiotic yogurt. But a decrease in serum levels of CRP was observed more in the PWLCD group s compared to PLCD and RLCD groups following 2mths of treatment. FOXP3, T-bet, GATA-3,TNF-α, IFNγ, TGFβ and RORγI genes expression was examined in peripheral blood mononuclear cells (PBMC’s) both before and after the intervention. RORγI expression was decreased in all 3 groups, while FOXP3 increased. GATA-3, TNF-α, TGFβ expression did not change. Though funnily T-bet gene expression got down regulated in all groups .Thus a suggestion that weight loss diet and probiotic yogurt had effects on gene expression in PBMC in overweight and obese individuals was given by the authors.38-40 In another study an 8weeks randomized, double blind, placebo and compliance controlled parallel study was done by Agerholm-Larsen et al in overweight and obese individuals to examine the effects of one strain of E. faecium and 2 strains of S. thermophilus.41 Randomly patients were divided in five groups;1)a yogurt fermented with 2 strains of S. thermophilus and 2 strains of L. acidophilus; 2) a placebo yogurt fermented with delta –acid lactone; 3) a yogurt fermented with 2 strains of S. thermophilus and one strain of L. rhamnosus ;4) a yogurt fermented withone strain of E. faecium and 2 strains of S. thermophilus and 5) 2 placebo pills daily.39 Following adjustment for small changes in body weight ,low density lipoprotein cholesterol (LDL-C) reduced and a significant increase in fibrinogen occurred after 8 wks in the 4th group i.e the one receiving a yogurt fermented with one strain of E. faecium and 2 strains of S. thermophilus as compared to the one getting chemically fermented yogurt and the placebo pill group. Further systolic BP got significantly decreased following 8weeks in group 4 the one receiving a yogurt fermented with one strain of E. faecium and 2 strains of S. thermophilus as well as in group 1 in contrast to group 3.41 Bifidobacterium, lactobacilli and S. thermophilus was given in the form of capsules to overweight subjects by Rajkumar et al. They found that this probiotic mixture, significantly improved the lipid profiles, decreasing total cholesterol (TC), triacylglycerol (TAG) and LDL-C levels while simultaneously increasing high density lipoprotein cholesterol (HDL-C) levels. Further this probiotic mixture, improved the insulin sensitivity along with decreasing C - reactive protein (CRP).42 While in a single blind parallel group intervention of 6week duration, 58 obese, premenopausal women were randomized into a daily intake of L. paracasei F19, flax mucilage or placebo. L. paracasei F19 did not alter any of the metabolic markers (like hemeostatic model of insulin resistance (HOMA-IR), Matsuda index, CRP and lipid profile compared with placebo.43 similarly intake of L. acidophilus. La 5 and B. animalis sub sp lactis Bb 12 did not affect HOMA-IR, BP, heart rate or serum lipid concentrations in over weight adults.44,45
Role of synbiotics
Effect of L. Rhamnosus CGMCCI 3274 with oligofructose and insulin supplementation was studied on weight loss and maintenance in obese men and women.46 Mean weight loss in women in the L. Rhamnosus group was markedly>than in women in the placebo group after the 1st 12 weeks, while it was similar in men in both groups. The L. Rhamnosus induced weight loss in women besides causing significant decrease in fat mass and circulating leptin concentrations also caused a relative increase of the bacteria of the L. achnospinaceae family In the faeces, a family that belongs to the Formicates phylum, a taxonomic group that is known to be positively associated with obesity.46 The examination of synbiotic supplementation on cardio metabolic risk factors, anthropometric profile, serum lipid levels along with oxidative stress levels in obese children was done in 2 studies. Synbiotic intake caused a significant decrease in the BMI-z- Score and waist circumference (WC), along with some cardio metabolic risk factors like TC, LDL-C and TAG.38,39 besides changes in anthropometric profile(percentage reduction as compared to baseline)were significantly> in children getting synbiotics. Total oxidative stress levels also got significantly reduced following synbiotic addition.47,48 Thus selected probiotics once added seem to benefit, BMI, WC, VFA, hip circumference in overweight/obese people. Further some probiotic strains alter the gene expression of particular transcription factors like down regulation of RORγI and up regulation of FOX-P3 in PBMCs, which was accompanied by beneficial changes in the immune system in overweight/obese subjects. Y et administration of L. paracasei F19 and L.acidophilusLa5 and B. animalis sub spp lactis Bb12 did not change the levels of inflammatory biomarkers. Some synbiotics decrease BMI in women; reduce fat massalong with serum leptin levels, increasing the Lachnospiraceae family in the faeces. Further synbiotic treatment help in reducing BMI-z–score and WC in children along with TC, LDL-C and TAG serum levels.
Role in insulin resistance syndrome (IRS)
Role of probiotics
Effects of Lactobacillus casei Shirota was examined in patients having IRS, to study gut permeability, presence of endo toxin and neutrophils function, insulin sensitivity index, quantitative insulin sensitivity check index, insulin sensitivity by oral glucose tolerance test, HOMA-IR and β cell function. Their was an increase in gut permeability, but no change in endotoxin and neutrophil function.49 In postmenopausal women IRS is a known risk factor for cardiovascular morbidity, like coronary heart disease and stroke. Boretta et al studied the efficacy of L. plantarium/placebo in postmenopausal women over a time span of 90 days. They found the TC, interleukin-6 (IL-6), gamma glutaryl transpeptidase (γ-GTP), levels markedly reduced in both groups at the end of the study, while LDC-C was signicantly low in the placebo group. Both glucose and homocysteine levels got signicantly lowered in the L. plantarium group as compared to placebo.50 Renorio-Jimenez et al conducted a double blind ,randomized ,crossover, placebo controlled and a single centre trial where 60 participants (18-65years), diagnosed with IRS was to be randomized in a 1:1 ratio to receive either a single daily dose of placebo or colony forming units of L. reuteri V 3401.This study has 2 intervention periods of 12weeks separated by a washout period of 6weeks and preceded by another washout period of 2weeks.The primary outcomes will be changes in lipopolysaccharide (LPS) levels at 12 weeks .Secondary outcomes will include anthropometric parameters ,lipid profile glucose metabolism, microbiota composition, hepatic steatosis and inflammatory and CVS biomarkers. Blood and stool samples would be collected at baseline, at midpoint (only stool samples) and immediately following each intervention period. Luminex technology will measure interleukins. Thus for the 1st time L. reuteri V 3401 will be evaluated in patients with IRS. Hence this study will provide valuable scientific information about the effects of this strain in IRS and metabolic syndrome patients.51
Role of synbiotics
Either synbiotic capsules having 7 strains along with fructo-oligosaccharide or placebo capsules for examining IR and lipid profile in patients having IRS were tried. Significant improvement in fasting blood sugar and IR was seen in synbiotic group.52 Thus some probiotic strains were of benefit by reducing the cell adhesion molecule-1 levels .Further in postmenopausal women, L. plantarium reduced TC, interleukin-6 (IL-6), gamma glutaryl transpeptidase (γ-GTP), glucose and homocysteine levels after 90days. Further the synbiotic mixture improved IR and HDL-C, while reducing the TAG and TC levels in subjects having IRS.
Role in type 2 diabetes mellitus (T2DM)
Role of probiotics
The effect of administration of probiotic soymilk containing L. plantarium A7 or soymilk alone was studied by Hann et al. This probiotic soymilk or soymilk alone was given daily as a supplement of their diet naturally consumed. Significant reduction in the level of promoter methylation in the proximal and distal MLH1 promoter region as compared to the baseline value. Also a significant increase in superoxide dismutase action was seen in the probiotic soymilk group as compared with the baseline value was observed. But no significant changes were seen in the promoter methylation of MSH2 within either group. Thus L. plantarium A7 inoculated soy milk might have anti oxidative properties, with the risk of mismatched base pairs in DNA among patients with T2DM.53 Administration of L. acidophillus La 5and B. animalis sub spp lactis BB12 was examined in T2DM patients. A marked difference between the groups concerning, mean changes in the HbA1c, TC and LDL-C levels was seen.54 Additionally an increase in HDL-C levels with a reduction in LDL-C/ HDL-C ratio was found in the treatment group.55 Earlier studies used the same strains in T2DM patients. A significant reduction in fasting blood glucose, TC, LDL-C and HbA1 c were observed, along with increase in erythrocyte superoxide dismutase and glutathione peroxidase activity and total antioxidant status as compared with the control group. Thus a conclusion was drawn that probiotic yogurt seems to be a promising agent for T2DM management, and might act as a functional food having both ant diabetic along with antioxidant activity.56,57 In a double blind, randomized study, males having T2DM,impaired or normal OGTT were put on a 4 week treatment course ,consisting of either L. acidophillus NCFM or a placebo to study the effects of oral probiotic supplementation on insulin sensitivity and the inflammatory response.58 The probiotic strain was found in 75% of the faecal samples, following treatment. Insulin sensitivity got preserved only in volunteers in the L. acidophillus NCFM group. Baseline inflammatory markers and the systemic inflammatory response was unaffected by the L. acidophillus NCFM supplementation.58 Further Razan poosh et al conducted a randomized double blind control trial in 60 patients who were assigned into 2 groups of 30 participants each to take either probiotic supplements or placebo for 6weeks. The probiotic supplements consisted of 7viable strains of Lactobacullus, Streptococcus and Bifidobacterium. Nutrient intakes were estimated using a 3day and 4hour dietary recall at the beginning and end of study. They found within group comparisons significant decrease in the levels of fasting blood glucose (FBG) (P=0.001) and increase in HDL-C (P=0.002).No significant changes were seen within and between group comparisons in the levels of insulin, triglycerides, TC, IR, weight, WC and BMI (allp>0.05).Thus concluding that a significant decrease in FPG level by multistrain probiotic supplements occurred within group comparisons though they advocated further studies to confirm results.59
Role of synbiotics
Multispecies probiotic supplement consisting of 7 viable and freeze dried strains and fructooligosaccharides was given to T2DM patients to study metabolic profile, CRP and oxidative stress in these patients. HOMA-IR increased significantly in both groups. But this increase was significantly more in the placebo group than in the probiotic group.Mean serum CRP were significantly lower in the patient group. Further probiotic supplementation=>to increased total glutathione levels compared to the placebo.60 In T2DM patients, clinical trials were done, to study the effects of synbiotic bread containing L. Sporogenes and insulin. Significant decrease in serum insulin levels, HOMA-IR, and homeostatic model assessment β cell function, serum lipid profile like TAG, TC/HDL-C occurred following synbiotic bread along with significant increase in HDL-C levels as compared to control bread.61,62 Another synbiotic shake that contained L. acidophilus, B. bifidum and fructo–oligosaccharides was evaluated regarding glycemic control and cholesterol levels in old people having T2DM. In this study TC, TAG, HDL-C along with blood sugar were examined. Only HDL-C increased significantly along with a significant decrease in fasting blood sugar but no change occurred in TC, along with TAG levels in the synbiotic group.63 Thus some of the probiotic changes seen in T2 Diabetics, were lowered fasting blood glucose levels, improved insulin sensitivity and an increased antioxidant status. Few synbiotics increased the total glutathione levels, HDL-C and decreased the fasting glucose levels and CRP. Further improvement in serum lipid profile was seen in T2DM patients consuming synbiotics.
Role in non alcoholic fatty liver disease
Role of probiotics
Aller R et al studied the effects of S. thermophilus and L. bulgaris on different liver function tests along with CV risk factors. A reduction in alanine amino transferees (ALT), aspartate amino transferase (ASP)and γ-GTP indicated improvement of liver function.64 In obese children having NAFLD, treatment with L. rhamnosus strain GG, caused a Significant decrease in the titer of anti-peptidoglycan-poly saccharide antibodies that are suitable as an indicator of SIBO. Further this randomized clinical trial also revealed a restoration of liver function through use of this Probiotics, showing a reduction in ALT.65 Using a Probiotics yogurt with L. acidophilus La 5 and B. lactis Bb 12 for 8weeks in NAFLD in a double blind randomized controlled clinical trial, a reduction in serum levels of ALT, ASP, TC and LDL-C occurred following L. acidophilus La 5 and B. lactis Bb 12 intake as compared to controls.66 Alisi et al carried out another randomized study ,where they found improvement of fatty liver severity significantly as examined by ultrasound ,along with a Significant decrease in the BMI of children having NAFLD after treatment with bifidobacteria, lactobacilli and S. thermophilus strain for4mths,suggesting that these strains might reduce liver fat and this prevent the progression of NAFLD.67 Alisi et al also examined glucagon like peptide 1( GLP1),an incretin secreted by cells of small intestine and proximal colon .They showed that the circulating levels of total and active form of GLP1 were significantly increased in the patients after4mths of treatment with synbiotics.67 Though not adequate data is available in humans ,it has been seen that treatment with probiotics improves the effectiveness of lifestyle modifications in obese subjects having NAFLD, might improve coventional LFT, and might reduce markers of lipid peroxidation.64–69 and NASH.70 Improvement in liver function may be secondary to a decrease in small bowel bacterial overgrowth(SIBO) and /or dysbiosis and thus a minor metabolic endotoxaemia in the host as once normal gut microbiota get reduced there may be a decrease in intestinal permeability.
Role of Synbiotics
Products derived from bacteria like lipopolysaccharides (LPS), ethanol and SCFA=>their arrival from intestine lumen to the liver. Also SCFAs stimulate synthesis and storage of hepatic triacylglycerols. This may=>saturation of the detoxification mechanisms =>accumulation of intrahepatic triglycerols (IHTG) content, thereby increasing the fatty liver severity. A randomized study using synbiotic made up of 5 probiotics (L. acidophilus, L. plantarius, L. delbruecki spp. bulgaricus, L. rhamnosus, B. bifidumand insulin)over 6mthsin adults having NASH, caused significant reduction in IHTG[70].That LPS produce pro inflammatory cytokines like tumor necrosis-alpha (TNF-α), that play a key role in IR and hepatic inflammatory cell recruitment in NAFLD. In a study done in 52 adults over 28wks, usage of symbiotic supplementation, that is a mixture of L. casei, L. rhamnosus, L. acidophilus, S. thermophilus, L. bulgaricus, B, breve, B. longum and fructo-oligosaccharides, showed that synbiotic supplementation inhibited NF-κ Band decreased TNF-α production.68The big limitation of this study was that the authors did not examine the gut microbiota to confirm the mechanism of action suggested. Further these results remain controversial as similar studies did not find significant changes in the values of TNF-α after treatment with different Probiotics64,65 and different synbiotics.67,70 respectively. A big difference in different variables seen in these studies, that included the intervention period, probiotic doses along with bacterial strains used along with the study subjects. Thus positive effects were produced by some probiotics, by improving liver function and decreasing SIBO. Regarding some synbiotics, a reduction in liver fat and TNF-α production, caused prevention of NAFLD. Further Porras et al.,71 reviewed how modulating intestinal microbiota in obesity related NAFLD by use of Probiotics, Probiotics, fecal microbiota transplantation works,71 (Figure1) (Figure 2). Despite these beneficial effects most of studies were of poor quality, from which conclusions can’t be drawn. However studies where there was a limited risk of bias, sample size was mostly small, with significant differences in the type and dosage of the prescribed probiotic, treatment duration and feeding type can be shown. The natural modification of the gut microbiota composition during the 1st periods of life and the role of external factors like diet and antibiotic consumption in inducing dysbiosis are poorly or not taken into account. In addition the results are usually conflicting .All these factors answer why pooling data to meta-analysis is difficult and hence results of various meta-analyses vary. Park and Bae carried out a meta-analysis of the studies based on use of Probiotics for weight loss that got published till dec 28 2014, excluding those enrolling pregnant women and infants. They selected 368 articles to begin with. But only 9 were randomized controlled trials (RCT), of which only 4 got included in the meta-analysis, since only in these studies means and SD regarding body weight was provided. Roughly 100subjects got treated with probiotics, while 100 got placebo. Changes in body weight, BMI and if possible VFM were studied. Nosignificant differences between groups were observed. Similarly a difference in VFM was not much. Hence the authors concluded that probiotics were ineffective in controlling weight changes.72 In another meta-analysis carried out by Borgeraas et al similar conclusions got drawn.73 In contrast markedly different results were reported in another meta-analysis done recently where studies until 2017 on the treatment of overweight and obese adults were considered [103].Of 8009 studies identified, 21 RCT got analyzed.
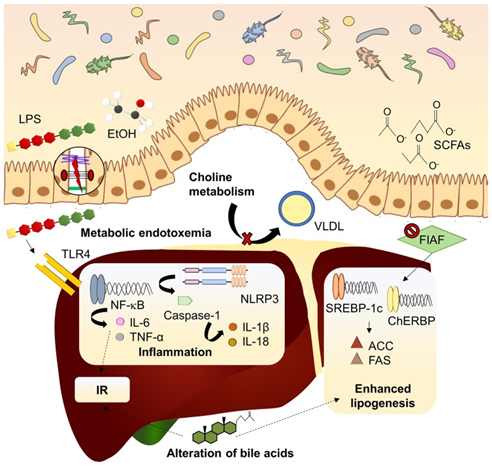
Figure 1 Courtesy ref 71-Mechanisms linking dysbiosis to NAFLD development. Dysbiotic gut microbiota is related to increased intestinal permeability and delivery of harmful substances (LPS, EtOH) to the liver, inducing inflammatory pathways mediated by PRRs. Inhibition of FIAF by IM promotes expression of lipogenic enzymes. Microbiota can modify bile acid pool in a mechanism associated to insulin resistance and lipogenesis enhancement. Choline metabolism is also affected by imbalanced microbiota, reducing lipid exportation through VLDL. ACC, acetyl-coA carboxylase; ChREBP, carbohydrate-responsive element-binding protein; EtOH, ethanol; FAS, fatty acid synthase; FIAF, fasting-induced adipocyte factor; IL, interleukin; IR, insulin resistance; LPS, lipopolysaccharide; NF-kB, nuclear factor kappa B; NLRP3, NOD-like receptor family, pyrin domain containing 3; SCFAs, short chain fatty acids; SREBP1-c, sterol regulatory element-binding protein 1c; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α; VLDL, very low-density lipoprotein.
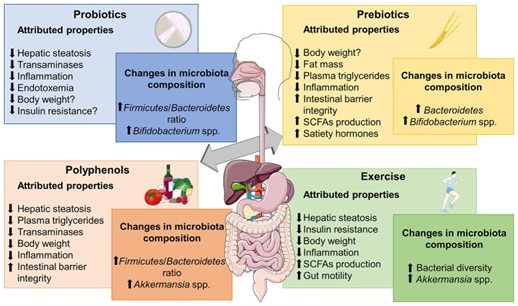
Figure 2 NAFLD Courtesy ref no-71-Metabolic effects frequently associated to different microbiome-based therapies for obesity-associated NALFD and relevant changes reported in microbiota composition. SCFAs, short chain fatty acids. The figure was made with use of Smart Servier Medical Art, licensed under a Creative Common Attribution 3.0 Un ported License.
Probiotics use was associated with a significant decrease in all parameters like weight; VFM. But data on probiotic usage was conflicting. Low dose was associated with a lower BMI decrease although VFM decrease was greater. Further a longer period of probiotics use even with low dose =>significant decrease of both weight and BMI. Thus Wang et al.,74 concluded that use of dietary agents for modulating gut microbiota was an essential tool for treatment of obesity.74 Yet different conclusions got drawn by Drer et al in another meta-analysis, where RCTs and crossover trials were taken into account and results were stratified by age.75 Studies involving pregnant women with term babies, neonates and individuals having GIT problems which might mask the effect of gut microbiota modulation were excluded. Starting from >1000 articles, 35 studies (14 in adults,7 in children and 14 in infants) were considered to have a relatively low risk of bias and were analyzed. Use of different Lactobacillus strains (2.7x1010cfu/day of Probiotics usage for 2-3mths was associated with significant weight loss .The amount of weight loss differed from study to study, although these variations were not considered to be dependent on type of probiotic used, intervention duration or characteristics of the baseline population. In children a significant increase in weight of the subjects receiving probiotics was seen as compared to control. Similar results were seen in infants receiving a probiotic enriched formula from the age of 3weeks to 10mths .Further data showing a potential positive effect on weight gain from probiotic usage has been published recently. Jones et al conducted a double blind, RCT, which was placebo controlled in 19 obese adolescents, where 3 packets/day of a mixture of Lactobacillus species (L. acidopophillus BA05, L. plantarium BP06, L. paracasei BP07, L. delbriecki sub sp bulgarius BD08), Bifid bacterium species (B. breve BB02, B.longum BL03, B. infantis BL04) and streptococcus thermopiles BT01 for 16weeks .In contrast to placebo adolescents who got Probiotics had significantly increased adiposity with no significant effects on gut microbiota, gut appetite regulating hormones, liver fat and fibrosis or dietary intake.76
The human gut is a place for trillions of bacteria that are collectively called gut microbiota. This universe ecosystem has evolved simultaneously with us and has a direct connection in the physiological processes, which might affect many organ systems like CVS, neural, immune and metabolic .In the past few decades research has made our understanding in the role of microbiota in energy homeostasis. There is evidence that bacterial strains are in a special equilibrium with obesity, but the question arises which microbial community is causally linked to obesity is still not clear. Although both in animal and humans it has been tried to correct gut dysbiosis by targeting gut microbiota, but the work is still in early stages and limited human data to make meaningful conclusions beyond simple association ,that has the risk of misinterpretations, or giving excess value to expected results when translating animal protocol to human trials. Future work is needed to understand how changes in the gut microbiota =>obesity or how obesity has an impact on changes in micro biome composition. The double interactions between the host and flora that includes genetic material exchange may hold the answer to meaningful clinical translation. More understanding of this complex crosstalk will help in the development of specially tailored along with targeted implementation of probiotic therapies. Further most of the earlier studies had been done in tightly controlled animal models that limit their potential application in humans subjects and whatever studies done in humans show limitations and contradictory results and need to be better stratified based on specific markers which consider lifestyle, age, genetics and other environmental influences on microbial composition. Understanding the metagenomic relationship between changing microbiota and probiotic species under different diets/nutritional status are needed. Most of research in this dynamic field has been done using Lactobacillus and, Bifid bacterium strains; hence there is necessity for finding new bacterial candidates along with their potential mechanistic effects on obesity. Till now clinical cohorts used have had small sample size and only focused on short term physical parameters, or inflammatory markers, which make long term studies highly important in future, work. Also randomized placebo controlled trials might help in developing guidelines for the use of Probiotics therapies in obesity and formulate nutritional recommendations besides addressing safety concerns regarding functional foods which contain Probiotics like fermented dairy products. Queries about specific bacterial strains‘s effect on bacterial composition, duration of therapy and appropriate doses still need an answer. But despite these pitfalls, probiotic therapy represents an exciting new avenue for medical treatment of obesity and associated metabolic dysfunctions, that we have been looking for.
None.
The authors declared there is no conflicts of interest.

©2019 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.