Advances in
eISSN: 2378-3168


Review Article Volume 13 Issue 1
College of Medicine, University of Science Arts and Technology, Montserrat, BWI
Correspondence: Orien L Tulp, College of Medicine, University of Science Arts and Technology, Montserrat, USA
Received: December 15, 2023 | Published: January 2, 2023
Citation: Tulp OL. Can caffeine activate your brown fat? Effects of caffeine, ephedrine, and norepinephrine on nonshivering thermogenesis in congenic lean and obese LA/Ntul//-cp rats. Adv Obes Weight Manag Control. 2023;13(1):1-6. DOI: 10.15406/aowmc.2023.13.00382
To determine the effects of norepinephrine (NE) or of caffeine (CAF) alone or in combination with ephedrine (EPH) were determined in groups of lean and obese LA/Ntul//-cp Rats, Body weights of obese were >> lean littermates (p=<0.01) and measures of RMR of lean > Obese (p=<0.05). The effects of caffeine, ephedrine, a caffeine+ephedrine combo, and the β- agonist epinephrine was examined. Caffeine (CAF) resulted in a 33% increase in VO2, ‘nonephedrine’ (EPH) a 48% increase, the combination CAF+EPH a 53% increase, and the NE a 33% increase in VO2. In obese rats, the increases in VO2 were of a similar percentage (21 vs 48 vs 47 % vs 31% for CAF, EPH, CAF+EPH and NE respectively although the peak responses attained in the obese tended to be of a significantly lesser absolute magnitude than were observed in the lean phenotype. The time to peak thermogenic response was similar in lean and obese phenotypes for each of the 4 treatment regimens, but the duration of the peak responses to each treatment differed between lean and obese phenotypes (Obese > lean) for CAF, EPH and the CAF+EPH combination but duration of the VO2 response was similar in both phenotypes for the NE treatment. Thus, these observations are consistent with a significant CAF-stimulated thermogenic response that was qualitatively similar to that of NE in both lean and obese phenotypes of the congenic LA/Ntul//-cp rat and which thermogenic responses were further augmented with EPH alone or in combination with CAF. Although the mechanisms of action of the pharmacologic and physiologic mechanisms elicited may differ among the three agents studied the results indicate that they are complimentary in nature in bringing about increases in parameters of nonshivering thermogenesis and thus increasing metabolic energy expenditure in both lean and obese rats. In conclusion, while caffeine as monotherapy may bring about limited weight loss, the combination of caffeine plus ephedrine was more effective in the lean and obese phenotypes of the congenic, non-diabetic LA/Ntul//-cp (Corpulent) rat.
In a recent editorial, it was proposed that coffee, a major source of caffeine, could activate one’s brown adipose tissue, stimulate parameters of fat mobilization and energy expenditure and thus contribute to effective weight control.1 Coffee and tea rank among the most widely consumed beverages in the world after water, and the morning coffee or afternoon tea are among the most widely experienced nutritionally related activities known to mankind. Caffeine can be found in over 60 species of plants and is used in several foods, drinks and over- counter and prescribed medications.2 Many healthful benefits have been ascribed to the consumption of coffee, tea, and other caffeinated beverages, ranging from mood boosters to performance enhancers, but can significant weight loss really be one of them? Thus, can a simple cup of the morning Joe really help to melt away those extra pounds as proposed, or is it just water chasing more water to result in the improved waistline? Is this the good news that really seems just too good to be true? Let’s turn to the lab rats to look for an answer and see how it correlates if at all with human studies. While we can’t conveniently or accurately determine the psychological or mood enhancing aspects of caffeine consumption in rats in our laboratory, we can determine if they experience any increase in metabolic rate, and hopefully gain some insight regarding potential weight loss after treatment with commonly available potentially thermogenic agents.
In animal studies, caffeine has been shown to increase measures of non-shivering thermogenesis in both lean and obese rats, and when combined with the ephedra alkaloid ephedrine, the combination has often been suggested as an adjunct for weight loss and improved control of energy balance.1–3 The caffeine and ephedrine combination in otherwise healthy individuals has been reported to result in moderate and often temporary weight loss, but it may be contraindicated in individuals with cardiovascular disorders.4 Caffeine and related methylxanthines occur as a constituents in many different edible foods, but are present in the highest concentrations along with numerous other chemicals in coffee and teas.5 Ephedrine is obtained from ephedra, which has been a constituent of the Chinese Medicinals formulary for thousands of years where it has been used to treat respiratory, mood and other disorders.6 Thus neither caffeine or ephedrine are newfound strangers to the global food supply market, the pharmacopeia or to human consumption. A summary of the three agents follows below:
Norepinephrine, a β-adrenoreceptor agonist
The established mechanisms of action of the three agents under study and discussion studies are physiologically complimentary. The common reference agent, norepinephrine, to which the other agents of this study are compared is a broad spectrum β-agonist, including stereospecific β1 and β2 actions in the cardiovascular and respiratory tissues, and β3 receptor affinity that is unique to brown adipose tissue (BAT) with corresponding increases in BAT-mediated nonshivering thermogenesis when activated by perturbations in diet and environment.7–9 Norepinephrine can also impinge on β1 and β2 receptors of smooth muscle tissues of the cardiovascular and respiratory systems, where it exerts cardio stimulatory responses, including dose related increases in the contractile force and heart rate (ionotropic and chronotropic responses) that may result in increases in pulse rate and cardiac output, in addition to peripheral relaxation of vascular smooth muscle. This norepinephrine can result in alterations in both systolic and diastolic blood pressure. It is noteworthy that the NE affinity for β2 receptors often predominates over that of β1 receptors in smooth muscle tissues, thereby enabling relaxation of bronchiolar smooth muscle and clinical improvement in respiratory disorders. NE along with epinephrine can also exert α1 and α2 receptor actions which can bring about vasoconstriction of vascular smooth muscle in addition to inhibition of presynaptic release of NE into the synaptic clefts where it can facilitate adrenergic actions in numerous peripheral tissues.7
Ephedrine, a sympathomimetic amine
Ephedrine, a sympathomimetic amine was derived from ancient Chinese medicine remedies containing ephedra where it was often used for relief from mood and respiratory disorders exhibits sympathomimetic properties in man and animals.1 The sympathomimetic amine exerts both direct and indirect sympathomimetic effects via direct activation of both α- and β-adrenergic receptors respectively. The indirect effects include inhibition of neuronal NE reuptake, thereby resulting in relative increases in NE release from synaptic vesicles that release the neurohormone. Thus, ephedrine administration and clinical use results in increases in neural norepinephrine release, where it impinges predominantly on α1- and β1-adrenergic receptors, resulting in pharmacologic responses resembling those of epinephrine although they tend to be somewhat attenuated when compared to epinephrine on a molecular basis.7
Caffeine and methylxanthines
Caffeine and methylxanthines primarily affect the actions of the cardiovascular, respiratory, adipose, muscle, renal, and nervous systems, albeit each with a different aspect of its physiologic or pharmacologic mechanism of action and physiologic outcome. Proposed mechanisms of action differ for different systems, however: In cardiovascular, respiratory and adipose tissues for example, the major action appears to be centered around a dose-dependent inhibition of phosphodiesterase activity, while in neural and other tissues, inhibition of adenosine receptors, antagonism of benzodiazepine receptors, and dysregulation of calcium translocation from intracellular storage depots play decisive roles, often affecting aspects of wakefulness, cognition, renal function and others.7,10
As mentioned above, the pharmacologic effects of caffeine and other methylxanthines largely center around their ability to inhibit phosphodiesterase activity in skeletal muscle, adipose tissues, and other tissues, where methylxanthines bring about increases in the intracellular concentrations and the intracellular half-life of cAMP, thereby enabling a prolongation of the duration of the cAMP actions. In adipose tissue phosphodiesterase inhibition results in promotion of lipolysis, followed by release of FFA and glycerol, which may be utilized as an energy source in multiple tissues, thereby decreasing reliance on glucose as a primary substrate in energy metabolism and a downward shift in the respiratory quotient (R.Q.)11 to values of less than 1.0. Caffeine also exhibits mild CNS stimulatory actions in part by interfering with aspects of adenosine metabolism in addition to its effects on enhancing wakefulness and quickening of physiologic response times. Caffeine occurs naturally in over 60 species of plants many of which are used in various beverages and other foods. In humans, it is metabolized into three distinct dimethylxanthines by hepatic p-450 actions to produce paraxanthine, which stimulates lipolysis; theobromine, which exerts diuretic and vasorelaxation effects, and theophylline, which can dilate airways and thus is often useful in alleviating respiratory conditions where it can improve blood pO2 saturation levels and the efficiency of respiratory exchange.12 However, caffeine is considered a fairly weak inhibitor of phosphodiesterase enzymes, and the in vivo concentrations at which behavioral effects occur may be insufficient to be associated with meaningful phosphodiesterase inhibition.13,14
The stimulatory effects of caffeine and methylxanthines in cardiovascular and bronchiolar tissues are much more prominent than those observed in behavioral comparisons, however. The magnitude of phosphodiesterase inhibition due to caffeine and methylxanthines may account for the proportional cardiostimulatory and antiasthmatic actions observed in a dose related manner. Evidence for this is apparent since nonxanthine phosphodiesterase compounds that can bypass the inhibitory step of the phosphodiesterase reaction can also become cardiac stimulants and bronchiolar and tracheal relaxants.15 Indeed, Brackett et all determined that the magnitude of the phosphodiesterase inhibition correlated more closely with the efficacy of the inhibition rather than impacting on the affinity for the adenosine receptors per se.16–18 In addition to the differing mechanisms of action, in vivo plasma concentrations associated with the actions in different tissues cover a broad range, from a low of 5–10 µM to effect mild CNS stimulation, up to 50 µM for antiasthsmatic and cardiovascular effects.14 Caffeine metabolism to paraxanthine, dimethylxanthines, uric acids, di- and trimethyl allantoin, and uracil derivatives occurs primarily in the liver, and are slowly catalyzed by hepatic P450 microsomal enzyme systems which represent the rate-limiting step in methylxanthine metabolism. Methylxanthines are cleared primarily through the kidney, but because caffeine is efficiently reabsorbed by the renal tubules, only a vanishingly small percentage is excreted unchanged in the urine with each cycle of renal clearance.19,20
Synergistic effects of caffeine and ephedrine
The synergistic effects of caffeine and ephedrine, individually and in combination, have been applied to weight loss regimens for several decades, often yielding moderate success. The synergism occurs in part due to the broad, interconnected and partially overlapping mechanisms of their pharmacologic actions which can bring about increases in metabolic fuel mobilization and substrate availability in peripheral tissues, while diverting fuel oxidation away from oxygen rich glucose toward the newly mobilized oxygen-poor triglycerides and fatty acids and residual glycerol generated during the process. The shift to oxidation of fatty acids vs. glucose in peripheral tissues results in corresponding downward shifts in the R.Q. since fatty acids are notoriously poor in oxygen content when compared to glucose or amino acids (R.Q.= ~1.0 for glucose, averages ~0.82 for aminoacids, but may be as low as ~0.70 or less for fatty acids and ketones).11 As the R.Q. becomes decreased with the shift in relative availability of metabolic fuels for cellular oxidation and energy generation, the respiratory drive may shift upwards thereby improving not only parameters of weight loss with corresponding increases in VO2, but also exerting a glucose and amino acid sparing effect on substrate oxidation. Over time the rate of weight loss for an individual is likely to occur only gradually as the energy content of the stored fat is considerable greater than is observed during the oxidation of carbohydrate or protein in concert with hyperinsulinemia and the insulin-linked antilipolytic effects in adipose tissue in addition to the protein sparing effects of hyperinsulinemia on protein turnover.21 As a potential added attribute, the mood stabilizing effects of the caffeine and ephedrine likely compliment and improve the psychological impact that may occur during dieting and attempted processes associated with weight loss.
Groups of congenic young adult female lean and obese female SPF/antibody-free LA/Ntul//-cp rats (n= 5 rats/phenotype, littermates aged 12 weeks of age), derived from original breeding stock provided by Dr. C.T. Hansen, Division of SMALL Animal Genetics, Veterinary Resources Branch, Bethesda, MD USA. All animals used in this study were females due to the less severe magnitude of insulin resistance and disordered magnitude of glucose intolerance that develops in female than in male obese animals of this strain.21 Animals were housed in plastic showbox cages, lined with one inch of pine shavings, and received Purina Chow (#5012) and house water ad libitum, at 22 ±1°C. and 50% RH on a conventional light cycle. Measures of resting oxygen consumption were obtained in animals after an 8 hour fast in a small animal respiratory apparatus as described previously.21,22 After obtaining a satisfactory RMR in quietly resting animals, lean and obese rats were administered caffeine (20 mg/rat, EPH., 200 µg/KG BW, s.c) or both agents together, and measures of VO2 continued for up to 45 minutes post injection. An additional group was administered a submaximal dose of the β-agonist norepinephrine, 100 µg/kg BW for comparison. All injections were made in the upper mid dorsal region and a minimum of 72 hours interval was scheduled between repeat or successive measurements of VO2. Data were expressed as ml O2/min/kg BW-0.75 to compensate for differences in body surface area as described by Kleiber and Wang et al.23,24 All measures of resting VO2 were determined in quietly resting animals at thermal neutrality for the rat (30°C).21,22 Animals were weighed at the start of the study and before and after each measurement of VO2.Core body temperatures following the removal from the thermogenesis chamber core body temperatures were assessed with a lubricated quick response YSI thermistor placed in the transverse colon, at a point that corresponded with a location directly beneath the midpoint of the base of the liver. Data were analyzed by ANOVA and Students ‘t’ test.25 This study was approved by the Institutional Animal Care and Use Committee.
Body weights of the animals are depicted in Figure 1 and indicate that the body weights of the obese animals were markedly and significantly greater (p=<0.01) than their lean littermates while consuming the same dietary and environmental regimen. The RMR and the thermic responses to CAF, EPH, CAF+EPH, and NE [caffeine, ephedrine, caffeine+ephedrine, and norepinephrine respectively] are depicted in Figure 2. The RMR of lean rats were significantly greater than the RMR of their obese littermates (p = < 0.05). All four treatments resulted in significantly greater VO2 in the lean phenotype, with the greatest increase following the CAF+EPH combination. In the obese phenotype, only EPH, the CAF+EPH combination, and the NE resulted in significantly greater responses, while the increase with the CAF only was modestly greater but did not reach a level of significance, possible to the small n (n=5) of that group. On interest, the time to maximal response in vO2 was similar in both the lean and obese phenotypes for all agents studied (p = n.s.). The maximal duration of the response time following injection was longer in the obese than the lean phenotype for CAF, EPH and the CAF+EPH combination, but was similar in both phenotype for NE (Figure 4).
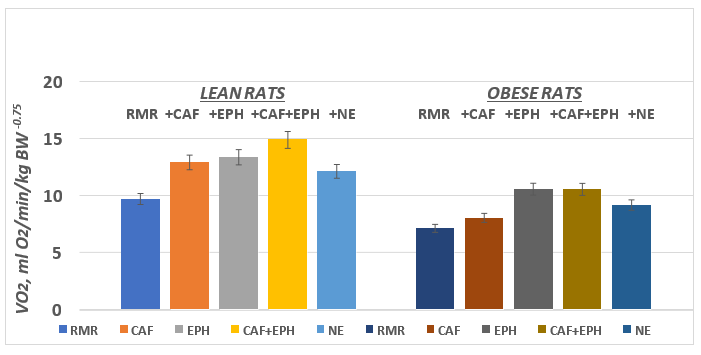
Figure 2 Effect of phenotype on thermogenic responses of rats. Data are mean ±1 SEM, n= 5 rats / group.
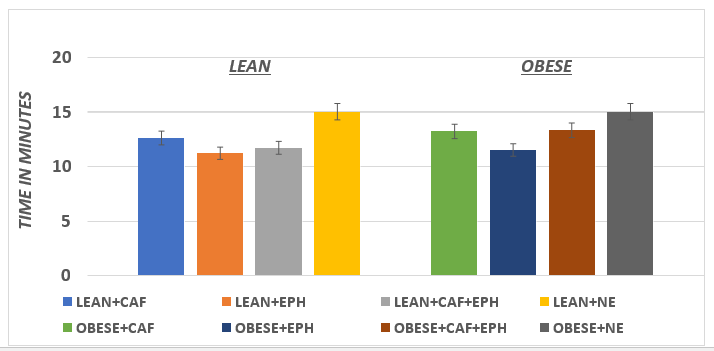
Figure 3 Effect of phenotype and treatment on time to maximal thermogenic response of rats. Data are mean ±1 SEM, n= 5 rats / group.
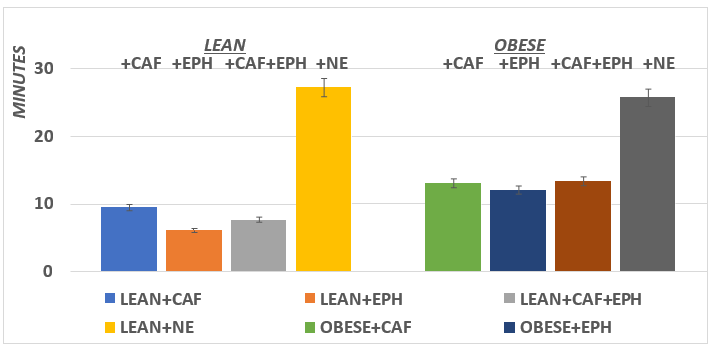
Figure 4 Effect of phenotype and treatment duration of thermic response of rats. Data are mean ±1 SEM, n= 5 rats / group.
Mean weight loss during one hour of RMR plus drug treatment is depicted in Figure 5. All treatments resulted in weight loss in both the lean and obese phenotype, but the greates absolute weight loss occurred in the obese phenotype. When expressed as a percent of initial body weight however, the average differences in weight loss were modest, as Obese vs. Lean weight loss averaged 126% for CAF, 123% for EPH and 119 % for the combination CAF+EPH. (p = n.s.) Measures of core body temperatures 30 minutes following administration of the thermogenic agents are depicted in Figure 6 and indicate that while the core temperatures following drug administration tended to be greater in the obese than the lean phenotype, the differences failed to reach a statistically significant level of difference (p = > 0.05).
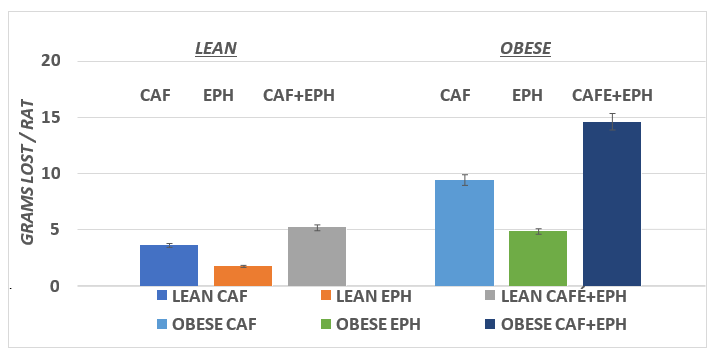
Figure 5 Effect of phenotype on and treatment on thermogenic body weight loss of rats. Data are mean ±1 SEM, n= 5 rats / group.
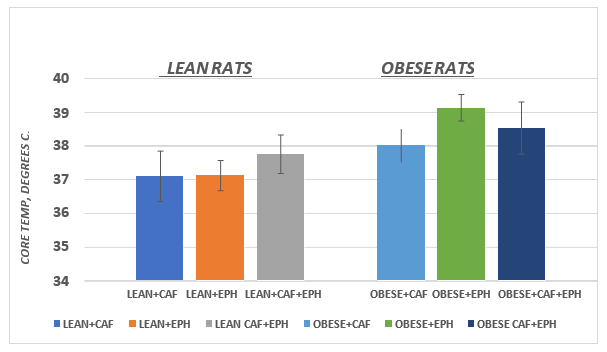
Figure 6 Effect of phenotype and treatment on core body temperatures of rats. Data are mean ±1 SEM, n= 5 rats / group.
The established mechanisms of action of the three agents under study and discussion are complimentary. The common reference agent, norepinephrine, is a β-agonist, including stereospecific β3 receptor affinity unique to brown adipose tissue.7,8 Norepinephrine also impinges on β1 and β2 receptors of smooth muscle tissues of the cardiovascular and respiratory systems, where it exerts cardio stimulatory responses, including dose related increases in the contractile force and heart rate ( ionotropic and chronotropic responses) that result in increases in pulse rate, cardiac output, in addition to peripheral relaxation of vascular smooth muscle and elevations in systolic and diastolic blood pressure. NE also exerts α1 and α2 sympathomimetic actions similar to but of a lesser magnitude than those of ephedrine.7
The results of the present study indicates that the caffeine+ephedrine blend resulted in significant increases in nonshivering thermogenesis, and in greater weight loss in the obese than the lean phenotype of young adult female corpulent rats. Increased heat generation via nonshivering thermogenesis, reflective of endogenous substrate oxidation from lipid stores in combination with other therapeutic parameters including dietary and lifestyle factors, is a primary goal of weight reduction therapies. The congenic LA/Ntul//-cp rat is an ideal animal model to investigate such research questions as the only known genetic difference between the lean and the obese phenotype in this strain is the epigenetic expression of an autosomal recessive trait for obesity, with all other variables remaining constant between similarly fed, housed and treated biological littermates.21,26 The origins of the corpulent rat strains have been discussed elsewhere, and while direct comparisons cannot be made between rodents and human subjects, the mechanisms of thermogenesis in addition to many pathophysiologic processes appear to be remarkably similar in the corpulent rat and human species.
Ephedrine is a sympathomimetic amine derived from ephedra that acts principally as a β-adrenoceptor agonist and has been used in traditional Chinese medicine primarily as a treatment for asthma and bronchitis, in addition applications in the treatment of depressive mood and other disorders for many years.1 Chemically the naturally occurring sympathomimetic amine occurs in two enantiomers of ephedrine of similar therapeutic efficacy as used in this study, and two pseudoephedrine enantiomers typically demonstrating lesser pharmacologic activity. In animal trials, ephedrine alone resulted in mean decreases in body weight of 14% and in a 42% decrease in body fat content over 6 weeks performed in studies utilizing MSG treated obese mice.27 The weight loss effects in that study were determined to have occurred mainly via a consistent 10% increase in daily energy expenditure.[ref] When the ephedrine was combined with caffeine, however, energy expenditure reached 20% above controls, body weight decreased by 25% and body fat by 75%, without notable alterations in food intake or lean body mass. The authors concluded that the decreases in body weight and fat content were primarily the result of the increases in thermogenesis rather than changes in energy intake per se, and after 4 weeks the previously obese mice achieved the weights and body composition of their normally lean counterparts. In contrast, energy balance was not significantly impacted by the methylxanthine administration when given as a monotherapy. In a study with LA/Ntul//-cp rats, the authors reported significantly greater thermogenic responses and daily weight loss with caffeine or a caffeine+ephedrine combination than with ephedrine alone in both lean and obese phenotypes, consistent with the murine studies cited above.3 In other studies in the same congenic rat model of obesity, responses to norepinephrine were typically greater in the lean than the obese littermates from early adulthood to older ages , suggestive of an impairment in the expression of nonshivering thermogenesis of undetermined origin in the obese phenotype.21,22
In a clinical trial, Acheson et al.27 investigated the effects of caffeine and coffee on the metabolic rate and substrate utilization in normal weight and obese individuals and observed that the metabolic rates increased for up to 3 hours following the caffeine ingestion in both treatment groups, while individual plasma insulin concentrations and parameters of carbohydrate oxidation remained unchanged. Plasma fatty acid concentrations nearly doubled, and lipid oxidation rates significantly increased during the 3 hours of observation. In addition, when caffeinated vs decaf coffee was included in a 3080 kj meal, the postprandial metabolic rates and markers of lipid oxidation were greater with the caffeinated vs the decaf coffee. The metabolic parameters were consistently of greater magnitude in the lean than the obese group however, suggestive that antilipolytic effects of hyperinsulinemia common among obese subjects likely may have contributed to the differences observed between the two treatment groups.
In another pair of studies, Dulloo et al.28,29 investigated the effects of a caffeine-ephedrine combination vs. ephedrine as monotherapy in lean and obese human volunteers and observed that the ephedrine/methylxanthine combination was twice as effective as ephedrine alone in increasing the fasting metabolic rate of both subject groups. The thermic effects to meals were decreased in the preobese and obese subjects, indicative of a defect in the thermic effect of feeding compared to normally lean control subjects. In addition, the combo treatment resulted in an overall 8 percent increase in 24-hour energy expenditure in the respirometer among the obese subjects, but the effects of the combination therapy on 24 hour energy expenditure were not found to be significant in the lean controls, suggesting that the caffeine-methylxanthine mixture could raise daily energy expenditure of those predisposed to obesity by normalizing their defective thermogenic response to food, and could be a useful adjunct in the treatment of obesity.
Astrup et al.4 investigated the effects of ephedrine with caffeine and β3 agonists on appetite and weight loss in adult female subjects and noted that the actions of the β3 agonists may be multifaceted, as they may mobilize thermogenic actions not only via β3 receptors unique to BAT but in other tissues.4,7 The global effects of the β3 agonists may be a reflection of the broad spectrum of β1-3 specific adrenoceptor actions in numerous tissues including liver and muscle, and thus capable of non-BAT mediated thermogenic activities associated with substrate oxidation and energy expensive processes linked to tissue biosynthesis. In those studies, the weight reducing effects of a caffeine+ ephedrine combination was also superior to placebo treatment or to the two agents as individual monotherapy during a study of 24 weeks duration. The authors noted significant anorexia in concert with increased thermogenesis among the caffeine+ephedrine group, also concluding that the combination of caffeine+ephedrine could represent a not only useful adjunct for the treatment of obesity but may also serve as a reference standard as newer β3 agonists may become available for clinical study.
The results of this study confirm the thermogenic actions of caffeine and ephedrine, alone or in combination on promoting thermogenesis in both lean and obese young adult littermate LA/Ntul//-cp rats, a congenic and SPF/ SAF model of genetic obesity. The findings are consistent with other human and animal studies of the thermogenic actions of caffeine, ephedrine, administered individually or in combination on parameters of thermogenesis and weight loss, and which presumably largely from fat mobilization and oxidation based on the known biochemical mechanisms of action of these agents which have shown increases in plasma FFA and glycerol secondary to phosphodiesterase inhibition and lipolytic actions following their administration. In other published studies, the lean mass was preserved when the above medicinal combination was administered, likely a reflection of protein sparing effects secondary to efficient lipid oxidation as a predominating energy source, and which enables a shift in energy utilization away from carbohydrates and protein status, thereby enabling their conservation. Endocrine dysregulation, particularly that of glucocorticoid and insulin also become markedly altered in obesity, leading to hyperinsulinemia and insulin resistance, which further impedes glucose uptake in peripheral tissues via inhibitory influences on GLUT4 transporters and glucose uptake in skeletal muscle and adipose tissue,31 peripheral tissues, as FFA uptake in peripheral tissues is an energy independent process, and is largely devoid of significant oxygen content in the saturated FA chains, resulting in a downward shift in the respirometry quotient, and helping to drive the respiratory VO2 that is reflective of parameters of energy expenditure.11
The findings reported in numerous clinical trials are consistent with the results reported in this study. In all studies reviewed, the combination of caffeine + ephedrine was superior to caffeine or ephedrine alone in promoting thermogenesis and weight loss. In addition, contrary to known cardiovascular precautions of administering adrenergic agents, none of the clinical studies reported significant adverse effects with the modest dosages administered. In pharmacology, it is commonly stated that ‘the dose makes the poison’ and adherence to that therapeutic standard effective weight loss may usually be obtained without incident with conservative dosages of a methylxanthine-ephedrine combination, ideally and as with all therapeutic protocols, under adequate medical supervision, and with special attention to the potential for unanticipated adrenergically-- mediated adverse cardiovascular events.
So, the question remains: Can coffee activate one’s brown adipose tissue, and expect to increase energy expenditure and lose weight? The answer is a solid both yes and no. No, because caffeine, the primary thermogenic ingredient of caffeinated coffee, lacks direct B3 agonist activity, required for the proper neuronal activation of brown adipose tissue. It can, however, increase the endogenous activity of the sympathetic nervous system indirectly, thereby increasing plasma norepinephrine and epinephrine, and thus although a less efficient and less direct mechanism, coffee and other methylxanthines can indirectly bring about an activation of BAT. This has recently been demonstrated by Velickovic et al,1 who detected evidence of the BAT-specific UCP1 thermogenic protein, following consumption of coffee.1 The UCP1 protein is also called thermogenin in earlier publications, perhaps a more descriptive moniker considering its origin and thermogenic action.32,33 The second answer to this question is an unqualified yes, as caffeine, like most other methylxanthines can among other actions, inhibit the intracellular enzyme phosphodiesterase that normally terminates the actions of intracellular cyclic AMP (cAMP). When present, cAMP results in the mobilization of FFA from stored triglyceride in both brown and white adipose tissues via activation of lipases, with subsequent availability for FA activation of thermogenesis in BAT in concert with increased FA availability for oxidation in muscle, liver and other somatic tissues that have the capacity to generate heat in response to the aerobic oxidation of oxidizable substrates. In summary, coffee and tea are akin to a medicinal cocktail of sorts, and are among the most medically safe, readily available, and widely consumed beverages the world over, and can likely bring about often sought after psychological, physiological, and medicinal advantages when consumed as directed. But if one really wants to if one really wishes to use coffee or tea for weight loss, it might be best to add an ephedrine chaser.
Supported by institutional resources.
The author declares no conflicts of interest.
None.

©2023 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.