Advances in
eISSN: 2378-3168


Research Article Volume 7 Issue 2
1Medical Corporation Doujinkai Kyoto Kujo Hospital, Japan
2Japan Bio Science Laboratory Co., Ltd, Japan
Correspondence: Vincent Hackel, Japan Bio Science Laboratory Co., Ltd., Japan, Tel 9259382732
Received: June 20, 2017 | Published: July 20, 2017
Citation: Ohnishi K, Hackel V. A two part randomized, placebo controlled double blind pilot study to determine the effect of ashitaba ( Angelica keiskei ) chalcone powder (chalCurb R ) on body weight and visceral fat in slightly obese adults. Adv Obes Weight Manag Control. 2017;7(2):242-246. DOI: 10.15406/aowmc.2017.07.00189
The Japanese plant ashitaba (Angelica keiskei) contains flavenoid chalcones, 4 hydroxyderricin and xanthoangelol has been shown to prevent aspects of metabolic syndrome. A two-part pilot study was undertaken to determine ashitaba’s effect on body weight, waist circumference visceral fat in overweight adults. There were two 8weeks randomized, placebo-controlled, double-blind parallel studies. Part 1: 15 healthy males and Part 2: 26 overweight adults. The treatment groups received 200mg/day ashitaba chalcone powder (8%) and control groups received a placebo daily. Weight, visceral fat, subcutaneous fat, total fat, BMI, waist circumference and body fat were measured at baseline, weeks 4 and 8. Subjective symptoms, blood tests, urine test, heart rate, blood pressure were conducted to determine safety. There were no significant differences between groups in primary endpoints or safety measures. There was a significant reduction of the visceral fat area and body weight in the treatment group at 8weeks compared to baseline particularly in those with the highest amount of visceral fat. A decrease in the visceral fat and body weight over 8weeks was significant only in the treatment group, suggesting future studies are warranted. The study supports the safety of the product.
Keywords: ashitaba chalcone, flavonoid, metabolic syndrome, visceral fat reduction
Medicinal plants are used throughout the world for multiple therapeutic purposes and are growing in popularity in the United States. A recent National Health Survey reports that nearly 18% of adults in the US used medicinal plant based supplements for a variety of reasons.1 The plant Angelica Keiski is native to Japan where it is known as ashitaba. This natural medicinal plant is primarily consumed in China, Japan and India as a health promoting agent, similar to green tea, food and medicine.2 The sap derived powder contains several flavenoid chalcones. The most physiologically active of these are 4 hydroxyderricin and xanthoangelol.2,3 These chalcones have demonstrated antioxidant, anti-inflammatory, antiangiogenic, antidiabetic and anti-obesity properties. Furthermore, they have been shown to inhibit platelet aggregation, induce apoptosis in cancer cells and suppress differentiation of preadipocytes to adipocytes.4–10. Xanthoangelol, one of the flavenoid chalcones may improve lipid metabolism in the serum and the liver resulting in decreased risk of atherosclerosis and suppression of hepatic lipid accumulation.9–12 Although ashitaba has been reported to possess numerous health benefits, it has most notably been utilized as a medicinal plant to prevent obesity and its complications, such as metabolic syndrome, specifically insulin resistance and hyperlipidemia. Numerous in vitro and in vivo studies support the use of ashitaba as an anti-obesity and anti-diabetic agent, although clinical trials are needed to confirm the relevance of these findings.3 For the aforementioned reasons and background, a two-part series of studies were undertaken.
Subjects were randomly assigned with 13 in each group (9 males, 4 females) to receive the intervention of 200 mg/day ashitaba chalcone powder or to receive a placebo for 56 days every evening after dinner. Subjects were tested at baseline, week 4 and week 8 (day 56). Subjects were asked to refrain from making any changes in diet, exercise or lifestyle behaviors for the duration of the study. On test days subjects were asked to report to the laboratory having fasted for at least 8 hours. To maintain standardization, all subjects kept a monitored diary for ensuring that the same dietary intakes were followed throughout the study. Primary outcome measures included weight and body fat area (the visceral fat area, the subcutaneous fat area and the total fat area) measured by CT scan. Secondary endpoints included BMI, waist circumference and body fat, measured by bioelectrical impedance (Omron Body Composition Monitor with Scale, "HBF-354 IT-2" (OMRON HEALTHCARE Co., Ltd.). Subjective symptoms, blood tests, urine test and physical exams (heart rate, blood pressure) were conducted to determine the safety of the product.
Statistical analysis included a between-groups (treatment group vs. placebo group) unpaired t-test on the change rate in endpoints at 8 weeks. For within group analyses, a one-sample t-test was done for values measured at each time period from the baseline. Significance was established for both the efficacy and the safety evaluation at 5%, one-tailed. Akihabara Medical Clinic Ethics Committee approved all aspects of this study (study #21565). This study was conducted in compliance with “Declaration of Helsinki” (Tokyo, 2004) and “Ethical Guidelines for Epidemiological Research” (Ministry of Education, Culture, Sports, Science and Technology. Ministry of Health, Labor and Welfare, 2004) and in accordance with the study protocol.
Visceral fat
There was a decrease in visceral fat area from baseline to 8 weeks in both groups, with the difference between the two groups was not significant, shown in Figure 3a. However, the amount of the reduction (i.e., the change amount and the change rate from baseline) was large and significant from baseline to week 8 (107.00±43.56 → 96.98±38.41 cm2, a reduction of 7.94%) in visceral fat area only in the treatment group (P<.05).
Age |
BMI |
% Body Fat |
P: 52.4 + 6.9 |
P: 26.59 + 1.08 |
P: 28.48 + 4.84 |
Table 1 Subject Information at Baseline
P: Placebo; A: Ashitaba
Total fat
Both groups showed a reduction in the total fat area at week 8, however, the change from baseline values was only significant in the treatment group compared to the baseline (330.27±59.57→311.12± 66.26cm2, a reduction of 5.99%), shown in Figure 3b.
Body weight
There was a reduction in body weight from baseline at week 4 and at week 8 in both groups, with significance only in the treatment group (baseline 73.86±6.15; week 4 73.14±5.98: week 8 73.05 ±6.21kg, a reduction of 0.81 kg at week 8), shown in Figure 4a.
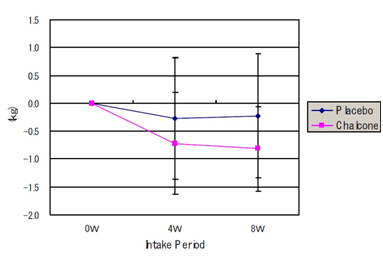
Figure 4a Body weight change
Weight: 0w (73.86±6.15kg), 4w (73.14±5.98kg) p<0.05, 8w (73.05±6.21kg) p<0.01
Body Mass Index (BMI)
Body Mass Index (BMI) decreased in both groups, the reduction was significant at week 4 and week 8 only in the treatment group (baseline 26.92± 1.36; week 4 26.67±1.41; week 8 26.64±1.45, a reduction of 0.28 at 8th week), shown in Figure 4b.
Waist circumference
There was a significant reduction in waist circumference at weeks 4 and 8 compared to baseline in both groups, shown in Figure 4c, but there was no significant difference between groups.
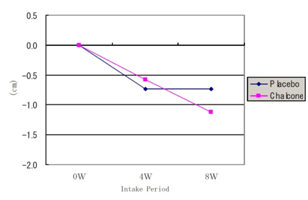
Figure 4c Waist circumference change.
Chalcone: 0w (94.48±3.84), 4w (93.90±3.67) p<0.01, 8w (93.35±3.88) p<0.01
Percent body fat
Percent body fat decreased at weeks 4 and 8 in both groups but was not significant. The subcutaneous fat area decreased at week 8 compared to baseline in both groups (3.21% in the treatment group; 1.13% in the intervention), however, there was no significant difference between groups.
Hip circumference
The hip circumference decreased at weeks 4 and 8 in both groups. It was significant at week 8 in the placebo group (Baseline → 8th week: 98.48±3.22→98.02±3.14cm, a reduction of 0.46cm at 8th week) and weeks 4 and 8 in the treatment group (baseline 100.07±3.2; week 4 99.61±3.23; week 8 99.38±3.20cm, a reduction of 0.69 cm at week 8.
Waist to hip ratio
The waist to hip ratio decreased at 4th and 8th week in both groups. It was significant at week 4 in the placebo group and at week 8 in the treatment group.
Safety
There were no significant changes within or between groups in any of the safety parameters tested. Within the confines of these studies and study designs, no serious adverse events or medically clinically significant changes were observed, indicating the safety of this botanical extract nutritional product.
In part two of this study, slightly obese adult males and females ingested a test food containing 200mg of ashitaba chalcone for 8weeks. There was no significant difference between groups.
However, a within-group analysis revealed a significant reduction of the visceral fat area and body weight in the treatment group at 8weeks compared to the baseline. This is similar to the trend revealed in part one of the two part study. Similarly, male C57BL/6 mice fed a high-fat diet with 0.01% ashitaba extract by weight for 16weeks showed lowered diet-induced body weight and body fat and lowered serum levels of glucose, insulin, and cholesterol when compared to the positive controls. It was suggested that ashitaba extract regulated lipid metabolism in adipose and liver tissue by activating AMP-activated protein kinase.9 In contrast, male Wistar rats fed a high-fat diet in combination with ashitaba powder at 17, 170, or 1700mg/100g body weight for 28days did not show significant differences in body weight gain, food intake, or relative organ weights when compared to the controls.13 In a study on six-week-old male stroke-prone spontaneously hypertensive rats SHRSP fed diets containing 0.02% or 0.1% xanthoangelol (0.02 and 0.10 Xan, respectively) for 7weeks, xanthoangelol was shown to improve lipid metabolism in the serum and liver, resulting in a reduction of the risk for development of atherosclerosis and suppression of hepatic lipid accumulation, parameters of metabolic syndrome.12
A study by Ohnogi et al.,14 was conducted to determine ashitabaʼs efficacy for treating metabolic syndrome. For this study, 9 human subjects ingested ashitaba juice comprised of dried leaves and stems for 8weeks. Following ingestion, all subjects had significantly lower visceral fat, body fat, and body weight at the end of the 8th week, and no adverse clinical changes were attributed to ashitaba. While this study reported similar results to those found in the current study, it lacked controls, and therefore must be interpreted with caution. The current study did not find a significant difference between the treatment and control but the trend points to a greater change in the treatment group suggesting that perhaps with a larger number of subjects or a longer treatment time a significant difference may emerge. In addition, in part one of this study, we found that the greatest changes in visceral fat occurred in subjects with the greatest amounts of fat at baseline. Perhaps the subjects in part 2 of our study were not overweight enough or did not have enough fat stored in the visceral region. Perhaps in future studies it can be incorporated into the study design to compare subjects of varying amounts of visceral obesity.
Ashitaba chalcone has a long history of human use as a dietary supplement with claims of multiple health benefits and with many published animal studies without toxic effects.2 For example, safety has been demonstrated in Wistar rats at a dose of 1700mg/kg.13 In addition, a safe dose of 300mg/kg has been reported in male and female rats.3 Similarly, this study did provide evidence for the safety of ashitaba chalcone at a dose of 200mg/day for 56days in humans.
In Conclusion, although no significant differences between treatment and control groups were determined, our results provide support for further research into the effects of ashitaba chalcone on aspects of metabolic syndrome such as body weight and visceral fat. Future studies may include a larger number of subjects with a greater degree of obesity, particularly with a greater degree of visceral obesity. In addition, our study provides support for the safety of 200mg/day ashitaba chalcone.
These two studies were 100% financed and supplied by Japan Bio Science Laboratory Co., Ltd (JBSL). The authors would like to acknowledge the work of Douglas Kalman PhD, RD and Susan J Hewlings PhD, RD of Substantiation Sciences LLC. For their work in writing, editing and submitting this manuscript.
Principal investigator Dr. Katsunori Ohnishi of Japan Bio Science Laboratory Co., Ltd. Supervised administration and management of the study, managed subjects, managed adverse events, ordered the study to the contract research organization, managed the quality of the test food, dealt with liability issues regarding the test food, supplied the test food and approved a study protocol. Vincent Hackel supervised the study from study design to publication.
The authors are employees of JBSL. The funding sponsor, JBSL, had final approval in the design of the study; in the writing of the manuscript, and in the decision to publish the results.

©2017 Ohnishi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.