Advances in
eISSN: 2377-4290


Case Report Volume 5 Issue 2
Staff Optometrist, Wilmington VAMC, USA
Correspondence: Rajni K Acharya, Staff Optometrist, Wilmington VA Medical Center, 1601 Kirkwood Highway WIlmington, DE 19805, USA, Tel 732-668-4973
Received: September 24, 2016 | Published: November 1, 2016
Citation: Acharya RK. Vision loss after cranial radiotherapy: case report and review. Adv Ophthalmol Vis Syst. 2016;5(2):231-234. DOI: 10.15406/aovs.2016.05.00151
Background: The ocular manifestations of various types of radiation vary from patient to patient. As the primary sites of damage are endothelial cells, co-existing microvascular diseases, such as diabetes and hypertension, increase the risk of developing retinopathy and maculopathy from radiation treatment. Prompt recognition of findings and initiation of treatment may delay or decrease associated vision loss.
Case report: A 62-year-old male presents to the emergency department complaining of persistent hiccups for three days. Further testing revealed right basal ganglia CNS lymphoma for which he received chemotherapy and external beam radiation. He was referred to the eye clinic from the oncology department with complaints of blurred vision in both eyes. His systemic medical history was remarkable for Insulin Dependent Diabetes Mellitus Type 2 and hypertension. On examination, the visual acuity was 20/40 in each eye. Fundus examination revealed cotton wool spots, macular edema and marked capillary non-perfusion in the posterior poles of both eyes. Over the course of a year, his vision deteriorated to 20/400 in each eye. Considering the patients good control of his underlying microvascular diseases, radiation retinopathy was considered likely etiology for the relatively rapid progression of retinal findings and concurrent worsening of vision. Despite a series of Intravitreal Avastin Injections administered in an attempt to stabilize vision, the patient’s vision was unable to be restored.
Conclusion: Extensive patient education is imperative for patients undergoing radiation treatment, due to ocular or periocular neoplasms, considering the various possible ocular manifestations. Coexisting microvascular diseases should be taken into consideration as they increase the risk of developing retinopathy. This case report reviews the histopathology, risk factors, natural course and long term sequelae of radiation treatment to familiarize the practicing eye care professional with contemporary evaluation and therapeutic considerations for this potentially vision threatening condition.
Keywords: radiation retinopathy, maculopathy, anti-VEGF injections, lymphoma, optic neuropathy, external beam radiation
Since the 1800’s, radiation has been utilized in the treatment of several types of cancers. Emil Grubbe was the first American physician to employ this treatment on a neoplasm in 1895.1 By 1933, the first signs of retinal injury following radiation were noted by H.B. Stallard.2 He noted hemorrhages, exudates, optic nerve head swelling, optic atrophy and retinal pigment epithelial changes in fundi of patients with treatment of various ocular neoplasms. As the primary site of damage are endothelial cells, co-existing microvascular diseases such as diabetes and hypertension increase the risk of developing retinopathy and maculopathy from radiation treatment. This case report highlights the ocular manifestations of radiation retinopathy (RR) resulting from external beam radiation involving the globe and ocular adnexa. The histopathology, risk factors, natural course and long term sequelae of radiation treatment are reviewed to familiarize the practicing eye care professional with contemporary evaluation and therapeutic considerations for this potentially vision threatening condition.
A 62-year-old male presented to the Emergency department complaining of persistent hiccups for three days that were accompanied with chest pain. Patient’s systemic history included Insulin dependent Diabetes Mellitus type II, Peripheral nerve disease, hypertension, chronic hepatitis C, Colonic polyps, primary CNS Lymphoma, Post-traumatic stress disorder, major depressive disorder, seizure disorder and lumbar radiculopathy. His active medications were listed as Atenolol, Dexamethasone, Diazepam, Insulin (Novolin), Levetiracetam, Lisinopril, Multivitamins, Sennosides, Triamcinolone acetonide cream and Zolpidem. Documented allergies include shellfish and strawberries.
This patient is an established patient at eye clinic with an extensive ocular history of mild non-proliferative diabetic retinopathy OU, talc retinopathy, Bell’s palsy, acute hypertensive retinopathy and recurrent clinically significant macular edema s/p focal laser OU. A year prior to the diagnosis of the lymphoma, he also had a complete palsy of the third cranial right in the right eye accompanied with a ptosis. MRI and MRA were used to rule out an intracranial aneurysm. Two years prior to that in 2009, the patient was diagnosed with a Posterior uveitis and vitritis in the left eye. After receiving two sub-Tenon Kenalog injections, patient’s vision improved from count Fingers to 20/30 OS.
A CT scan was performed at his initial presentation to the emergency department on March 10, 2012. The scan showed a 2.5 x 2.5 cm mass in the region of the right basal ganglia with extensive surrounding edema that extended into the brainstem. Patient was immediately sent to a local hospital where he was diagnosed with a primary Central Nervous System Lymphoma (PCNSL). He was treated with chemotherapy (high dose Ara-C and Methotrexate and external beam radiation to the entire brain (whole-brain radiation therapy or WBRT). Six month after initiation of treatment, he was referred to the eye clinic from the oncology department with complaints of blurred vision.
His entering acuity was 20/40 in OD and OS with habitual glasses. No improvement was found with a new refraction. He had an APD in the left eye that had been longstanding since the posterior uveitis. Extraocular muscles were full and smooth OU. His lids showed dermatochalasis OU and mild ptosis OS (consistent with history of Bells palsy). Slit lamp examination of the anterior segment revealed mild blepharitis OU, pingueculae nasally OU, clear cornea without any staining OU, deep and quiet anterior chamber without cells and flare OU with angles graded as 4 by Von Herrick. Irides were round and reactive without rubeosis OU. Posterior segment showed multiple CWS and scattered intraretinal hemorrhages OD>OS that were attributed to diabetes. No evidence of neovascularization, vasculitis, retinitis, pars planitis or choroiditis was noted. Reduction in vision was contributed to h/o focal laser treatment OU. No treatment was indicated at this time and patient was scheduled to return to clinic in 4 months.
Due to concurrent chemotherapy treatments, the patient was not able to make it to his follow up. He finally presented 6 months later in April 2013, when his vision had reduced to 20/80 OD and OS. At this time, his left APD optic nerve was pale, macular edema was noted in OD and the retinopathy was still present OU. Fluorescein angiogram ordered at that time also showed marked capillary non-perfusion in both eyes, predominantly in the posterior pole region OD. Pan retinal photocoagulation was recommended at this time, however, it was contraindicated due to poor patient cooperation and limited cognitive abilities (Figure 1&2).
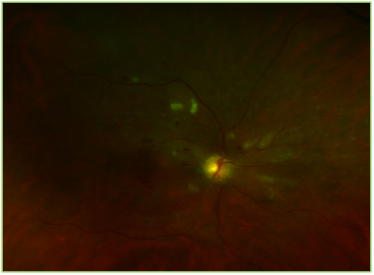
Figure 1 Optos image of the right eye showing multiple cotton wool spots, flame shaped and intraretinal hemorrhages and focal laser scars.
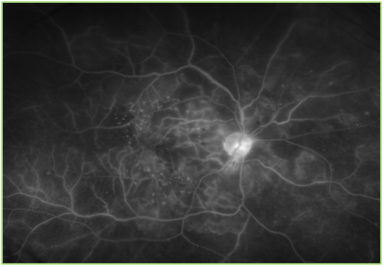
Figure 2 Optos image of the fluorescein angiogram of the right eye. Focal laser scars, microaneurysm, hemorrhages and marked capillary non-perfusion can be noted, especially in the posterior pole.
Patient returned few months later in July 2013 for a follow up with an entering acuity of 20/400 OD and OS with recurrent cystoid macular edema OD only and pale optic nerves OU. Intravitreal Avastin injection was administered in OD to decrease the macular edema. No visual improvement was noted by this treatment but the edema had fully resolved by next visit. At this time, it was decided to administer Avastin injections in both eyes to decrease the sequelae of radiation. Even after three injections in OD and two in OS, there was no significant improvement noted in visual acuity. As seen in the OCT findings from above, the foveal contour is disrupted and the macular thickness has significantly decreased. Over the course of two years after the initial radiation dosage was administered, patients’ visual acuity decreased from 20/40 to 20/400 OD and OS. Due to the optic neuropathy and notable macular ischemia present in both eyes, vision was not able be restored (Figure 3).
Cancerous tumors are commonly treated with radiation therapy because of its ability to control growth. During this procedure, DNA of the cancerous tissue is damaged, leading to cellular death. According to the International System of Units (SI), the unit used to measure ionizing radiation is called gray (symbol: Gy). The total dose of radiation is fractionized so the normal cells have time to recover since the tumor cells are less capable of repair between cycles.3 The three main divisions of radiation therapy are differentiated by their radiation source: external beam radiation therapy (EBRT), brachytherapy and systemic radioisotope therapy. The source of EBRT is outside of the body where brachytherapy uses sealed radioactive sources placed exactly in the area of treatment. Systemic radioisotopes are a form of target therapy that is either infused into the bloodstream or ingested.3
Chemotherapy is sometimes used as an adjunct for the treatment of certain types of tumors. Study done by O’Neill et al.,4 has shown that combined modality therapy with chemo and radiation did not produce an overall survival advantage. Subsequently, a long-term survival study performed by Abrey et al.,5 showed that combined modality therapy did improve survival, but relapse was common. They also noted that severe damage to the nervous system (neurologic toxicity) was a significant complication, especially in patients over the age of 60. Results of this include changes in behavior, cognitive functioning, dementia, and balance and coordination problems. Similar to neurotoxicity, WBRT has substantial impact on ocular health. Incidence of retinopathy increases steadily at doses higher than 45 Gy, lowest reported dose being 11 Gy. RR has been reported within 6 months and up to 8.5 years after initiation of treatment.6 Our patient was treated with EBRT over the course of one year. Fraction size and total dosage is unknown as the treatment took place in another hospital.
The ocular manifestations of radiation treatment are very well documented and appear to be secondary to damage of the vasculature of the retina, choroid, and optic nerve. These include glaucoma, cataract, optic neuropathy, maculopathy, retinopathy, epiphora, dry eye and ectropion.7 Cataract may result after low doses of radiation to the lens of the eye and can easily be corrected through surgical treatment. Dry eye is a resultant of damage to the lacrimal gland during radiotherapy. Macular ischemia and optic neuropathy are more serious complications that may result in irreversible vision loss. Fortunately, severe optic neuropathy after cranial radiation is uncommon at low doses. Optic neuropathy is further broken down into optic papillopathy and retrobulbar optic neuropathy, due to its anatomical location. In its acute state, signs of optic papillopathy include ischemic whitening of the retinal nerve fibers entering the optic nerve, edematous optic disc swelling, circumpapillary exudative subretinal fluid and retinal edema, and linear hemorrhages on and around the optic disc.8
Retinopathy portrays clinical features such as cotton wool spots, intraretinal hemorrhages, microaneurysms and hard exudates. Histologically, the walls of arteries and capillaries thicken which lead to loss of endothelial cells.9 Damage to the vasculature will cause capillary closure with capillary non-perfusion becoming very prominent on a fluorescein angiogram, as seen in our patient. As a result of such ischemic conditions, neovascularization can occur, which in turn can cause vitreous hemorrhages and retinal detachment.10 Finger and Kurli have described stages of radiation retinopathy in regards to the clinical signs, symptoms, location, and best method of visualization and the risk of vision loss. The (Table 1) is included below.11 According to this classification, our patient was at stage 1 at initial presentation and progressed to stage 4 within one and a half years after initiation of treatment. On his fluorescein angiogram, marked retinal ischemia greater than 5 disc areas was present. Risk factors that increase the chances of developing RR include Diabetes, Hypertension, simultaneous chemotherapy, older age and pregnancy. Coexisting microvascular diseases, such as diabetes and hypertension, increase the risk as the primary site of damage is endothelial cells. In comparison, diabetes will cause more microaneurysm formation and initial loss of pericytes where radiation retinopathy will lead to early loss of endothelial cells.7
Laser photocoagulation is a widely used treatment for proliferative retinopathy caused by radiation. Chaudhuri et al.,12 reported a case in which full regression of neovascularization of the disc and retina was seen just two weeks of panretinal photocoagulation. Intravitreal triamcinolone acetonide is also an approved treatment of macular edema that can result in decreased retinal thickness and improved visual acuity. The mechanism behind its success includes down regulation of VEGF, inhibition of arachidonic acid pathway and reduction of blood-retinal barrier breakdown. Case reported by Hong et al. showed that intravitreal triamcinolone acetonide injections were successfully able to decrease macular edema and improve visual acuity in a patient with RR from radiation therapy of breast cancer that metastasized to the brain.13 In our case, Intravitreal anti-VEGF injections were used, as laser photocoagulation was not an option for this patient due to their physical and cognitive constraints. A report by Finger & Chin,14 showed that intravitreal bevacizumab improved or maintained vision, and reduced hemorrhage and retinal edema. Due to the limited amount of research on treatment guidelines, further clinical trials should be performed to establish whether early PRP, Intravitreal steroid and anti-VEGF injections would be valuable in decreasing the onset of radiation induced retinopathy.
Depending on the location of the tumor, patients may only have two choices: probable death from the underlying disease or possible blindness as a complication from the treatment. Most patients are willing to accept the later. Patients being treated with radiation, to ocular or periocular neoplasms, should be made aware of these possible complications. Coexisting microvascular diseases should be taken into consideration as they increase the risk of developing RR. Laser photocoagulation, intravitreal steroid injections and anti-VEGF injections are used as treatments to potentially improve vision and decrease the chances of vision loss. However, further clinical studies need to be performed to establish proper guidelines of treatment. As neurotoxicity can significantly impact cognitive functions, options for treatment become limited. From this case, we conclude that once macular ischemia and optic neuropathy have developed as a result of radiotherapy; there are very low chances of vision recovery.
None.
Authors declare that there is no conflict of interest.

©2016 Acharya. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.