Advances in
eISSN: 2377-4290


Case Report Volume 2 Issue 2
Los Angeles VA, Ambulatory Care Center, USA
Correspondence: Kenneth Phi, Los Angeles VA Ambulatory Care Center, 351 E Temple St Optometry 112C, Los Angeles, CA 90012, USA, Tel 7204722717
Received: January 20, 2015 | Published: March 5, 2015
Citation: Phi K. Uveitis-Glaucoma-Hyphema (UGH) syndrome-a complex complication. Adv Ophthalmol Vis Syst. 2015;2(2):44-47. DOI: 10.15406/aovs.2015.02.00036
Uveitis-Glaucoma Hyphema syndrome is a condition involving cataract extraction complications. It is important to understand this rare complication of cataract extraction in order to preserve a patient’s vision. Misdiagnoses of UGH syndrome can cause excess testing and may eventually lead to loss of vision. This case report reviews the management of Patient 0 from the Illinois Eye Institute who suffered from UGH PLUS, details of his disease process along with clinical management employed throughout a 4-month span are described.
Keywords: uveitis-glaucoma-hyphema syndrome, iatrogenic, cataract extraction, misdiagnosis, ellingson syndrome
UGH, uveitis-glaucoma-hyphema; IOL, intraocular lens; ACIOL, anterior chamber intraocular lens; PCIOL, posterior chamber intraocular lens; GCA, giant cell arteritis; NVI, neovascularization of the iris; YAG, yttrium aluminum garnet; OD, right eye; OS, left eye; HbA1c, hemoglobin A1C; PEE, punctate epithelial erosion; IEI, illinois eye institute; QD, 1 time a day; BID, 2 times a day; QID, 4 times a day; gtt, drop; ER, emergency room; VA, visual acuity; PMMA, polymethyl-methacrylate; PO, by mouth; RTC, return to clinic; PPV, pars plana vitrectomy; PCP, primary care physician; IPUGH, incomplete posterior uveitis-glaucoma-hyphema; PBK, pseudophakic bullous keratopathy; CME, cystoid macular edema
Uveitis-Glaucoma-Hyphema Syndrome (UGH syndrome, or “Ellingson” Syndrome) is a rare condition caused by mechanical iris trauma-generally by a malpositioned/subluxed intraocular lens (IOL).1 The IOL can be a posterior chamber or anterior chamber intraocular lens. UGH syndrome generally causes a decrease of vision in the affected eye and can develop immediately or over the course of years. On occasion, there is a total loss of light perception which indicates additional pathology.2,3 As in uveitic glaucoma, the patient can experience pain. The affected eye can appear injected and the patient may report seeing a reddish tint covering their vision.4 Upon diagnosis of UGH syndrome, increased intraocular pressure, blood/clotted blood in the inferior anterior chamber angle, and cell/flare in the anterior chamber may present in the involved eye; hence, the name Uveitis-Glaucoma-Hyphema. Nonetheless, diagnosis is not always simple. Vision loss and pain can easily be mistaken for other issues such as Giant Cell Arteritis (GCA) or amaurosis fugax due to the transient monocular vision loss. It is important to consider UGH syndrome in the differential diagnosis in order to avoid loss of time and unnecessary testing. Other signs can include neovascularization of the iris (NVI) or corneal edema (due to the intraocular lens being prolapsed and in contact with the corneal endothelium). Treatment of UGH syndrome involves explantation or repositioning of the misplaced/subluxed lens.3,4 Rarely, if an iris blood vessel is found to be the source of bleeding, an argon/YAG laser is employed to stop the active bleed.5 Ultimately, UGH syndrome is a complication of cataract extraction and/or head trauma.
Patient 0 was a 52 year old African American male who presented to the primary care clinic in the Illinois Eye Institute on April 25th, 2013 for a comprehensive eye exam in order to evaluate blurry vision OD>OS. He described the condition as significantly painful OD. He also described being given unknown eye drops, which did not help his symptoms (red cap, blue cap, and gray cap). It had been 6 years since his last IEI eye exam and 4 months since cataract surgery at an outside hospital. His symptoms began at an unknown time interval after his cataract surgery. Patient was a poor historian. He had been previously diagnosed with proliferative diabetic retinopathy and hypertension. Patient is on 10+ medications for these conditions, including aspirin. He reported an allergy to benoxinate/fluorescein sodium.
Patient mood and orientation were both abnormal. Blood pressure taken in-office measured 180/90 mmHg for this 240 lb, 73” male. Uncorrected distance visual acuity was 20/600@ 6ft OD with no improvement on pinhole and 20/50-1 OS. His pupils were fixed and irregular OD with a 1+ reaction OS. Patient’s confrontational visual field was constricted superiorly OD and constricted inferiorly OS. Slit lamp examination showed edematous lids/lashes OU, conjunctival 1+injection OU with 2 exposed sutures OD, 2+diffused PEEOD, 1+OS on the cornea along with a temporal CE scar OD. Angles were open OU via Van Herick method. Anterior chamber examination showed 4+plasmoid cells & flare with a vitreous prolapsed at 5+7 o’clock OD and a deep and quiet anterior chamber OS. Neovascularization of the iris was noted OD and an unremarkable iris OS. ACIOL placed in OD with a nuclear sclerotic and cortical cataract OS. Intraocular pressures measured 21 mmHg, 19 mmHg OD, OS via Goldmann Applanation Tonometry with proparacaine+fluorescein and lid holding. A fundus exam revealed hazy views with vitreous cells OD and optic nerve showed a cup to disc ratio of 0.50, macula was flat and intact, artery to vein ratio was 2:3, and peripheral retina was flat and intact 360 OD, OS. Patient was to discontinue his blue/gray/red drops given by the other doctor. Atropine 1gtt OD was instilled in-office and the patient was to begin prednisolone QID OD for presumed chronic iridocyclitis. Patient was instructed to return to the clinic in 4 days with a glaucoma specialist.
Follow up #1–April 29th, 2013 (Primary Care Clinic)
The patient reported the symptoms of pain and blurry vision constantly. However, some relief was given by the prednisolone QID. VA was reduced to light projection and no improvement from pinhole OD. OD pupil remained fixed. Upon confrontational visual fields he was unable to see fingers and had full range of motions on extraocular muscle testing while noting pain on eye movement. Upon slit lamp examination, there was trace conjunctival injection, NVI, 2+ cell & flare with inferior blood in the anterior chamber along with a vitreous prolapsed touching the cornea at 7o’clock. Intraocular pressure was 30 mm Hg, 21 mmHg OD, OS by tonopen. All other anterior signs remained the same as the previous visit. Post-dilation, the prolapsed vitreous was notedas obstructing the view to the posterior pole. A B-scan was performed and findings were clear except for the vitreous hemorrhage. A consult with a retinal specialist was requested for evaluation of the vitreous hemorrhage with chronic iridocyclitis. Also, patient was to continue on prednisolone 1% QID OD and start Combigan® BID OD. Patient was instructed to return to see the glaucoma specialist in one month.6
Follow up #2–May 21st, 2013 (Glaucoma clinic)
Patient 0 presented with a “throbbing” right eye explained to have “extreme pain, 10/10” localized temporally OD. He reported going to the ER the night before and was given morphine for relief. He also stated that there was a film in his right eye. His eye pain and inability to see had caused him to have 2 falls, once the night before and once the morning of the visit. The fall caused an injury to his chin. Patient presented with bloody, torn clothing, and was barely able to communicate with clinician. His VA measured as light perception OD. OD pupil was fixed at mid-dilated. During the slit lamp examination, most findings were stable and similar to last visit. However, his IOP increased to 47 mmHg, 15 mmHg OD, OS. Additional in-office medication was provided: 1 drop of Combigan®, 1 drop of Iopidine®, and 1 dropAzopt® were instilled 10 minutes apart, OD. Diamox was not given due to patient’s history of kidney failure. After 1 round of the in-office drops, pressure maintained at 48 mmHg OD after 30 minutes. Consent was given and one 25 mg tablet of Diamox was administered PO in-office with 1 drop Iopidine OD. OD was also cyclopleged with one drop of atropine for patient comfort. He was to continue prednisolone 1% QID OD, Combigan® BID OD, start Azopt® BID OD, atropine QD OD and RTC in 2 days for an IOP check in Urgent Care. A retina consult was scheduled to be in 9 days.
Follow up #3–May 23rd, 2013 (Advanced care clinic; retina)
Patient presented in urgent care reporting that the condition is not any better with complaints of pain, redness, photophobia, tearing, discharge, discomfort, and shortness of breath. Patient reported good compliance on taking prednisolone, Combigan®, Azopt®, but has not obtained atropine from the pharmacy yet. Patients VA was light perception OD and similar entrance testing results as the last follow up. Slit lamp examination revealed microcystic corneal edema, diffuse 3+ PEE, the same anterior chamber findings, except newly noted that the lens OD was a poorly fitting ACIOL touching the cornea and the iris. Intraocular pressures were 47 mmHg, 14 mmHg OD, OS. After dilation, the 2+ vitreous hemorrhage was noted again and all other findings appeared normal (Figures 1−3). Additional diagnostic testing was ordered for exterior/slit lamp photos, which were interpreted as a poorly fitting ACIOL OD with hyphema and NVI. The retinal surgeon performed an intravitreal injection of Avastin® 1.25 mg to regress neovascularization of the iris and to stabilize/improve the eye. Post-injection IOP via tactile measurements were now estimated to be 21 mmHg, 21 mmHg OD, OS. After the injection, the assessment of proliferative diabetic retinopathy with UGH syndrome OD was made. The plan stated to proceed with glaucoma/cataract specialist with ACIOL removal and IOP management.
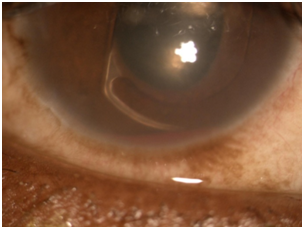
Figure 2 10x Slit lamp photo of malpositioned ACIOL with haptic rubbing on iris tissue causing hyphema.
Follow up #4–May 28th, 2013 (Advanced care clinic; glaucoma)
Upon this examination, slit lamp exam showed large temporal subconjunctival hemorrhage, 3+injection, and exposed sutures, OD. The cornea still had diffuse microcystic edema with the ACIOL haptic touching the cornea superiorly. Anterior chamber measured a 0.5 mm hyphema and the iris had several blood clots and inactive NVI. IOPs during this visit were back to 44 mmHg, 16 mmHg OD, OS. Dilated exam still showed vitreous touching posterior cornea at 7 o’clock. The glaucoma surgeon assessed vitreous hemorrhage with UGH syndrome OD. They reviewed medications, wrote instructions, and recommended sleeping propped up for resolution of hyphema. The doctor expressed a need to obtain previous surgical records. The decision was made to hold off on surgery until eye has quieted. Once quiet, the patient can proceed with ACIOL removal and anterior vitrectomy OD. The patient is to follow-up with glaucoma specialist in 1 week.
Follow up #5–June 4th, 2013 (advanced care clinic; glaucoma)
Patient reported minimal improvement in pain with significant photophobia. Patient was very tired, slurring words, and difficult to understand. Pain had been keeping patient awake through the night. Entrance testing and slit lamp examination revealed similar results from previous visit. IOPs during this visit were 36 mmHg, 20 mmHg OD, OS. The specialist sent an urgent request for surgical record. The patient remained in significant pain. The specialist recommended vitrectomy by retinal surgeon with ACIOL removal vs repositioning at time of PPV. The goal of the surgery was to reduce pain with little hope of improving visual acuity. Surgery was scheduled for June 12th, 2013. Patient provided with surgical clearance request from PCP and patient expressed understanding.
Surgical operation–August 14th, 2013 (University of Chicago)
Notes unavailable, however, retinal surgeon performed 23-gauge Pars Plana Vitrectomy along with an ACIOL repositioning and endo-laser OD to treat the vitreous hemorrhage and malpositioned ACIOL. Patient’s post-op medication regimen included Pred Forte®, Vigamox®, Isopto Hyoscine®, and Combigan® OD.
1 Day post-op–August 15th, 2013 (advanced care; retina)
VAs improved to finger counting OD at follow-up exam. Patient’s IOP now 32 mmHg, 15 mmHg OD, OS by Tonopen. Impression stated that the condition is improving with excellent post-op course.
1 Week post-op–August 23rd, 2013 (advanced care; glaucoma)
VA OD during this visit was light perception. Patient had an edematous cornea and IOPs of 16 mmHg, 12 mmHg OD, OS. Written instructions of medications provided for patient.
In 1949, Harold Ridley placed the first lens made of polymethyl-methacrylate (PMMA). In the 1950s, rigid ACIOLs were used in cataract extractions. Bullous keratopathy, inflammation, and cystoid macular edema were very common complications during this time. Iris fixed lenses were used in order to avoid contact with the angle.7 Today, PMMA, acrylate, and silicone lenses are often used. While PMMA has worked since the beginning, acrylate and silicone allow a smaller incision, which surgeons often prefer.5 Many choices exist for implantable lenses, from toric lenses to multifocals and accommodating IOLs. There are advantages and disadvantages to selecting each lens design. Single piece acrylic lenses allow for easy insertion into smaller incisions while its large haptics do not provide the best fit in the sulcus.5 Plate haptic lenses can be beneficial because the incision is small and allows for an easy insertion, but (like the single piece acrylic lens) it does not fit well in the sulcus. The 3 piece lens design will fit well in the sulcus while requiring a larger incision.7 Undoubtedly, surgeons will prefer certain lenses depending on their technique in the operating room and the patient’s anatomy. Overall, it is important to recognize the pros/cons of the lens designs and adapt that to the patient at hand.8
Prior to operation, ophthalmologists should note factors that may affect the difficulty of the surgery: zonular laxity, small pupil, medications (e.g. Tamsulosin®/anticoagulants), patient’s ability to lay flat, co-existing conditions (e.g. glaucoma/diabetes)…etc. These characteristics can become risk factors for the 1% risk of complications that cause decreased vision post-op.7 UGH is merely one of the many complications that can occur during post-op cataract surgery. Variations of UGH syndrome include UGH Plus and IPUGH (Incomplete Posterior UGH). IPUGH is a less severe variation defined as bleeding into the posterior chamber with/without glaucoma and no uveitis.3 Patient 0 actually classifies for the assessment of UGH Plus, which is defined as UGH syndrome plus a vitreous hemorrhage.9 Not much data/research currently exists about the complete pathogenesis and disease processes of these conditions at the moment.
Uveitis-Glaucoma-Hyphema occurred in Patient 0, causing near total loss of vision in his right eye. The patient’s extensive systemic history made it very difficult to identify this diagnosis. While, the subluxation of the ACIOL may have occurred soon after the cataract surgery, it is important to realize that the subluxation may have occurred when patient had multiple falls requiring a visit to the ER. Regardless, UGH syndrome is often a very difficult diagnosis secondary to its severe inflammation and difficulty to view structures within the eye. Ultimately, constant very high intraocular pressure is likely to cause permanent damage to the optic nerve. Therefore, it is important to manage the high IOP optimally. In the case of Patient 0, Diamox was provided in-office as a last resort when the pressure had not decreased with all other mechanisms. Originally, UGH syndrome was discovered as a result of excessive lens movement (due to small lens size or lens dislocation), poorly manufactured edges, iris-clipped IOLs, or rigid closed looped haptics. In a retrospective review by University of Florida, Gainesville, 97 patients were studied who had some form of UGH and 54% had an ACIOL while 34% had an iris-fixed lens. UGH syndrome can occur in cases with PCIOLs; however, it is much less likely due to the added stability provided by the lens capsule. Unfortunately, UGH syndrome can cause many complications (many of which were present in Patient 0-demarcated with an asterisk*) including pseudophakic bullous keratopathy (PBK)*, corneal staining*, chronic inflammation*, glaucomatous nerve damage, and cystoid macular edema (CME).3 In conclusion, it is important to remember the severe effects, signs, and management course for UGH syndrome. A severe and fairly rare complication of cataract extraction, UGH syndrome includes the classic triad of uveitis, glaucoma, hyphema. The hyphema may obscure the doctor’s view of the anterior chamber, posterior chamber, and IOL. In this setting, an IOP check and B-scan ultrasound may be useful. Ultimately, explantation of the IOL is often necessary as part of the therapeutic approach.10
None.
Author declares that there is no conflict of interest.

©2015 Phi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.