Advances in
eISSN: 2377-4290


Research Article Volume 8 Issue 6
1Department of Ophthalmology, Comenius University, Slovak Republic
2Department of Nuclear Physics and Biophysics, Comenius University, Bratislava, Slovak Republic
3Department of Stereotactic Radio surgery, St. Elisabeth University College of Health and Social Work, Bratislava, Slovak Republic
4Department of Medical Physics, Slovak Medical University, Slovak Republic
Correspondence: Alena Furdova, Department of Ophthalmology, Faculty of Medicine, Comenius University, Bratislava, Slovak Republic
Received: October 27, 2018 | Published: November 8, 2018
Citation: Furdova A, Waczulikova I, Sramka M, et al. Relative survival rates and presence of complications in uveal melanoma patients after stereotactic radio surgery. Adv Ophthalmol Vis Syst. 2018;8(6):283-289. DOI: 10.15406/aovs.2018.08.00322
This study presents findings from a retrospective analysis of 168 patients with uveal melanoma treated by stereotactic radio surgery (SRS) at linear accelerator in Slovakia. One-day session radio surgery at LINAC accelerator is a effective treatment modality in patients with uveal melanoma. The median tumor volume at baseline was 0.3cm3 (with range from 0.05 to 2.6cm3). The therapeutic dose was 35.0Gy by 99% of DVH (dose volume histogram). Average overall survival after stereotactic irradiation was 96.4% at 1year, 92.3% at 2years, 82.7% at 5years, followed by relatively stable survival of 81.6% during the rest of follow-up (6-10years). Survival rates at 5-year interval and the rates of secondary enucleating due to complications after one-day session linear accelerator irradiation were comparable to those achieved with other irradiation techniques used for treatment uveal melanoma. Radiation complications can lead to visual acuity reduction or secondary enucleating. Radiation-induced optic neuropathy (RION) is a severe ocular complication developing in high-risk patients with uveal melanoma after SRS. We analysed association between the secondary enucleating and the presence of secondary glaucoma or hemophtalmus as well as of the radiation-induced optic neuropathy after SRS. Secondary glaucoma led to secondary enucleating in 16.7% patients. The presence of optic neuropathy per se was significantly associated with a higher dose on the optic nerve (P=0.0123 in invariable and 0.0049 in multivariable analysis, respectively). Importantly, the overall survival of patients who underwent secondary enucleating was not different from the survival of patients without enucleating (P=0.7501).
Radiotherapy (external beam, proton beam, charged particle, brachytherapy, Leksell-gama knife, stereotactic radiotherapy) has become the preferred method of treatment for the vast majority of patients with uveal melanoma in the past decades. Different radiation techniques are nowadays used in treatment of uveal melanoma.1 Stereotactic radiosurgery (SRS) of head tumors - extra cerebral lesions, has been invented in the last twentyyears for treatment of small and middle stage posterior uveal melanoma. It provides good local control, with survival rates comparable with those achieved by other treatments.2‒5
Relatively infrequent approach to treatment of intraocular tumor is a one-day session (one fraction) LINAC radiosurgery.6 The images taken by a contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT) are aligned to same coordinate system so that information obtained from the fused images can be passed into a coordinate system used for treatment planning. A single fraction of 35.0Gy administered with a spatial accuracy using a collimating system is used. Neurosurgeon, ophthalmologist, medical physicist and radiotherapist all are responsible for SRS planning scheme. The process of the planning is based on the data acquired on CT and MRI images. Precise planning is very important for determining the stereotactic coordinates of radiation beams that are going to be applied into targeted tumor mass while trying to avoid critical structures (lenses, optic nerves, chiasm), because it can lead to reduction of visual acuity or other complications.6 Technique of one-day session SRS of uveal melanoma includes immobilization of the affected eye, which is achieved by surgical fixation of the eye globe by extra ocular direct four muscles to the stereotactic Leibinger frame. The stereotactic frame is fixed to the head and the sutures are tied to the stereotactic frame. Afterwards, the patient goes to CT and MRI examination with the immobilized eye to the frame.7 Linear accelerator-based stereotactic fractionated photon radiotherapy is another option, but an effective method to treat uveal melanoma with good local control rates and a 2year visual acuity retention rate comparable to brachytherapy (BRT) or proton beam radiotherapy. This technique is available also in small centres.8
Uveal melanoma is the most common and the most aggressive type of intraocular tumor in adults. In the United States the mean age-adjusted incidence is approximately 4.3 new cases per million of population per year, the incidence in Slovakia is 0.2 to 0.6/100 000 inhabitants.9‒11 Enucleating in patients with uveal melanoma was the standard treatment up the early 1970s, but other treatment modalities with the goal of preserving the eye have become increasingly popular. Furthermore, as diagnostic methods have improved, misdiagnosis rates have correspondingly declined. Ophthalmological examination and other modern diagnostic tools (ultrasound, optical coherence tomography, CT, MRI, positron emission tomography (PET/CT) have led to significant advances in the ability to diagnose primary uveal melanoma. Age and volume (size) of the tumor have been shown to be prognostic indicators following therapy for uveal melanoma.12 Uveal melanoma metastasize most frequently haematogenously to the liver.13‒15 Uveal melanoma is biologically and clinically distinct from cutaneous melanoma. Despite usual success in achieving local control, about 50% of uveal melanoma patients will develop metastatic disease. Gene expression profiling has improved the ability to stratify high-risk patients, but outcomes for patients with metastatic disease remain incredibly poor. The therapeutic advances that have translated into improved patient survival in cutaneous melanoma have, unfortunately, not yielded similar benefits in advanced uveal melanoma.
In this study we aimed to analyse overall survival of the patients with uveal melanoma treated by SRS. Radiation-induced optic neuropathy (RION) is a severe ocular complication in patients with uveal melanoma after SRS, therefore, besides from assessing the overall survival, we also analysed relationship between the outcome-secondary enucleating-and the preceding factors, secondary glaucoma or hemophthalmus, and the radiation-induced optic neuropathy after SRS.
We analysed data in a retrospective study of 168 patients with intraocular uveal melanoma stage T1 to T3 who underwent SRS at C LINAC. Patients’ protocol included tumor stage, maximum elevation of the lesion, volume, localization, but also general status, age, gender, and functional tests, such as visual acuity and perimeter.
The SRS treatment planning protocol was optimized according to the critical structures-lenses, optic nerves, and chiasm (Figure 1). Planning protocol was done by fusion of CT and MR scans. SRS was performed by one-day session on linear accelerator Model LINAC C 600C/D Varian with 6MeV X. Tumor volume calculation was based on the ROI (region of interest) of the tumor, the planned therapeutic dose into the tumor mass was 35.0Gy by 99% of DVH (dose volume histogram).
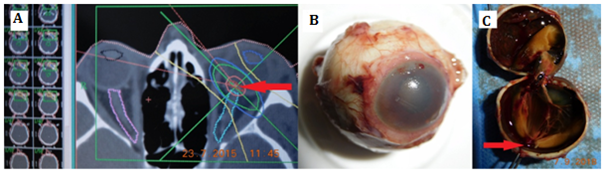
Figure 1 (A)Stereotactic planning scheme in a patient with small choroidal melanoma near to the optic disc), (B)enucleated eye globe due to secondary glaucoma and hemophthalmus, (C) enucleated eye globe – arrow shows the reduced tumor mass.
Patients were followed in 3months interval by an ophthalmologist (biomicroscopy, ophthalmoscopy, aplanation tonometry, ultrasound, optical coherence tomography, fundus photo) and MRI findings. Patients who passed SRS are checked regularly by their oncologist to screen metastasis in 6months interval. This check-up includes abdominal ultrasound, liver’s function test, and chest X-ray or PET/CT (Positron Emission Tomography/Computed Tomography) examination.
The event-free or disease-free interval was defined as the time from treatment until the event in question, or the development of metastases, respectively. Patients were seen in three-month intervals in the first year after the SRS, later in six-month intervals following SRS; 5years after SRS they were asked to come regularly every year for complete examination.
Statistical analysis
The demographic and clinical characteristics of patients were analysed using descriptive statistics. Two-sample t-test or alternatively Mann-Whitney test was used to test for between-group differences. Categorical variables are presented as counts and relative frequencies and differences between two categorical variables were tested by chi-square or binomial tests.
Kaplan-Meier survival curves accompanied by the log-rank test results were used to compare distribution of events (e.g. secondary enucleating or death) by the selected grouping variable. Cox proportional hazard analysis was performed to identify significant clinical variables and their contribution to prediction of secondary enucleating and overall mortality.
All P‐values were considered significant at a two‐tailed P‐value of <0.05.
Descriptive characteristics
A group of 168 patients with uveal melanoma (147 choroidal melanoma 87.5%, 21 ciliary body melanoma 12.5%) treated at linear accelerator LINAC were reviewed. Patient age ranged from 20 to 92years with a median of 63years. Median tumor volume at baseline was 0.3cm3 with range from 0.05 to 2.6cm3. Median of maximal dose applied was 49.0Gy (range from 37.0 to 51.0Gy). The range of follow-up was from 24months to 11years.
Tumor volume reduction after irradiation in 6months interval after SRS was followed from 6 to 110months interval after irradiation by MRI, ultrasound examination and photo documentation. Tumor local control was successful in 95% of patients in 2years interval after SRS and in 75% of patients in 3years interval after SRS.
Late complications like macular destruction, maculopathy, optic nerve atrophy, retinopathy, cataract, bleeding into vitreous (hemophthalmus), and secondary glaucoma were observed (Figure 1). Twenty two patients (13.1%) developed maculopathy (79% of the total of 28 enucleated eye globes). Secondary enucleating (28 patients) was necessary due to secondary glaucoma, in 4 patients with large tumors due to progression, and in one patient with a small tumor due to patient´s preference for surgery. Histopathologically uveal melanoma spindle cell type A was confirmed in 20 patients (71%), melanoma spindle cell type B was found in 2 patients (7%) and patients melanoma epitheloid type in 6 (22%).
Average overall survival after stereotactic irradiation was 96.4% in 1year, 92.3% in 2years, 82.7% in 5years, followed by relatively stable survival of and 81.6% during the rest of follow-up over 6-10years (Figure 2) (Table 1). Tumors were divided according to tumor volume groups - small–less than 0.5cm3, middle – 0.5 to 1.0cm3 and large–over 1.0cm3. In the group of small-to-medium tumor patients who developed metastasis, the average time-period after irradiation was 22.6monthsyears and in the group of patients with large melanoma who developed metastasis, the average follow-up from irradiation was 14.5months. Survival estimates over the 1 to 10year follow-up of patients with uveal melanoma treated by SRS in Table 1.
Follow-up |
Patients at risk |
Deaths |
Survival (%) |
Lower 95% confidence limit (%) |
Upper 95% confidence limit (%) |
0 |
168 |
0 |
100 |
n.a. |
n.a. |
1 |
162 |
6 |
96.43 |
92.22 |
98.38 |
2 |
127 |
6 |
92.24 |
86.7 |
95.53 |
3 |
92 |
8 |
85.76 |
78.68 |
90.63 |
4 |
66 |
5 |
80.75 |
72.57 |
86.71 |
5 |
43 |
4 |
74.64 |
64.71 |
82.15 |
6 |
31 |
2 |
70.91 |
59.99 |
79.35 |
7 |
22 |
0 |
70.91 |
59.99 |
79.35 |
8 |
12 |
0 |
70.91 |
59.99 |
79.35 |
9 |
5 |
0 |
70.91 |
59.99 |
79.35 |
10 |
1 |
0 |
70.91 |
59.99 |
79.35 |
Table 1 Survival estimates over the 10-year follow-up of patients with uveal melanoma treated by SRS
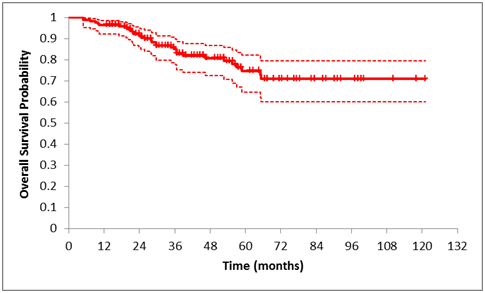
Figure 2 Kaplan-Meier curve of overall survival of the whole group of patients with uveal melanoma treated by SRS. Estimates for each year are presented in Table 1.
Bivariable and multivariable analysis of overall-survival and its predictors
Statistically significant independent risk factors for death were: higher age, higher initial volume (volume at the time of diagnosis) and, as expected, with the presence of metastases (Figure 2). No other type of complication per se was significantly associated with overall mortality risk (Table 2). Statistically non-significant risk factors for death in bi variable analysis were: male gender and other clinical characteristics, such as age, volume, and metastases presence (Figure 3).
|
Survivors (137/100%) |
Dead (31/100%) |
Total (168/100%) |
probability |
|||
|---|---|---|---|---|---|---|---|
N |
column % |
N |
column % |
N |
column % |
||
Glaucoma |
39 |
28.50% |
9 |
29.00% |
48 |
28.60% |
0.9999 |
Hemophthalmus |
12 |
8.80% |
3 |
9.70% |
15 |
8.90% |
0.9999 |
Optic neuropathy |
19 |
13.90% |
1 |
3.20% |
20 |
11.90% |
0.1285 |
Maculopathy |
43 |
31.40% |
6 |
19.40% |
49 |
29.20% |
0.2733 |
Cataract |
40 |
29.20% |
10 |
32.30% |
50 |
29.80% |
0.8282 |
secondary Enucleation |
22 |
16.10% |
6 |
19.40% |
28 |
16.70% |
0.6044 |
Metastases |
3 |
2.20% |
16 |
51.60% |
19 |
11.30% |
< 0.0001 |
Table 2 Amplitude in mill volts of the Lead-1 of electrocardiography in sheep
*Significant (P≤0.05); NSNot significant (P>0.05)
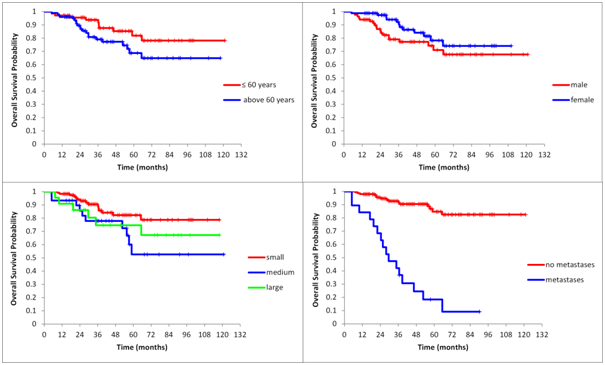
Figure 3 Unadjusted Kaplan-Meier curves of overall survival stratified by age (P=0.082), gender (P=0.131), volume (P=0.041) and by presence of metastases (P<0.0001).
The results of bi variable analysis (higher age vs. lower age, P=0.082; male vs. female, P=0.131; small volumes vs. larger volumes, P= 0.041 and presence of metastases, P<0.0001) were confirmed by multivariable analysis (age P=0.0006; gender, P=0.2004; tumor volume, P=0.0015 and presence of metastases P<0.0001).
Complications and their association with the need of secondary enucleating
Further, we were interested in the relationship between the secondary enucleating and the possible risk factors - late complications due to irradiation such as opticoneuropathy, hemophthalmus, or secondary glaucoma. Complications and their association with the need of secondary enucleating (Table 3). The results of analysis of the risk for secondary eye loss due to complication leading to secondary enucleating due to complications (especially the presence of hemophthalmus and glaucoma) had significantly higher rates
|
Without enucleation (140/100%) |
With enucleation |
Total |
Effect of event |
Effect of dose |
|||
|---|---|---|---|---|---|---|---|---|
N |
column% |
N |
column% |
N |
column% |
probability |
probability |
|
Glaucoma |
25 |
17.90% |
23 |
82.10% |
48 |
28.60% |
< 0.0001 |
0.62432 |
Hemophthalmus |
9 |
6.40% |
6 |
21.40% |
15 |
8.90% |
0.0215 |
0.2372 |
optic Neuropathy |
17 |
12.10% |
3 |
10.70% |
20 |
11.90% |
0.9999 |
0.0123 |
Maculopathy |
43 |
30.70% |
6 |
21.40% |
49 |
29.20% |
0.3713 |
0.0016 |
Cataract |
41 |
29.30% |
9 |
32.10% |
50 |
29.80% |
0.8218 |
0.0006 |
Table 3 Amplitude in mill volts of the Lead-1 of electrocardiography in sheep
*Significant (P≤0.05); NSNot significant (P>0.05)
In 23 patients (82% of enucleated patients) secondary enucleating was necessary due to developed secondary glaucoma, in 4 patients with large tumors due to progression, and in one patient with a small tumor due to patient´s preference for surgery. A group of 19 patients (11.3% of the total sample) developed metastasis. Enucleating free interval ranged from 3months to 8.2years.
The nonsignificant result for optic neuropathy was, partly, due to the fact that its effect was implicitly included in the association between glaucoma and enucleating (Table 4).
Variable |
Probability |
Hazard ratio |
95% confidence interval |
Age |
0.8449 |
1.0027 |
0.9761 to 1.0301 |
Male gender |
0.2398 |
1.4661 |
0.7747 to 2.7745 |
Initial volume |
0.0000 |
5.6952 |
3.1340 to 10.350 |
Choroidal/ciliary body |
0.4089 |
0.7446 |
0.3697 to 1.4994 |
Optic neuropathy |
0.0396 |
2.9708 |
1.0531 to 8.3799 |
Table 4 The outcome secondary glaucoma detected within follow-up three years (significance of the Cox model: P<0.0001). The presence of optic neuropathy per se was significantly associated with higher dose on the optic nerve (P=0.0123 in bivariable and 0.0049 in multivariable analysis)
The individual Kaplan-Meier plots show development of the events leading to secondary enucleating and, as a result, to lowering risk of death for enucleated patients. First, the development of optic neuropathy presumably associated with irradiation (Table 3) second, significant association of glaucoma on the presence of optic neuropathy (Figure 4 upper plot; P=0.034), third, significant association of secondary enucleating on the presence glaucoma (Figure 4 middle plot; P<0.0001), and, finally, the non-significant difference in the overall survival between enucleated and non-enucleated patients (Figure 4 lower plot). However, the overall survival of patients who underwent secondary enucleating was not different from the survival of patients without enucleating (P=0.7501).
For medium to large uveal melanoma tumors or those in a location that may not be amenable to plaque brachytherapy, proton beam radiotherapy or stereotactic irradiation at linear accelerator can be used. These techniques can be considered for treatment of tumors surrounding the optic disk and fovea, where brachytherapy (plaques) cannot be placed directly. Due to the physical properties of charged particles, specifically the sharp decline in radiation dose beyond the targeted area, and collateral damage to normal ocular tissue is reduced. As a result, a high rate of local tumor control (> 95% at 15years) can be achieved without significantly worse complications than plaque brachytherapy. Studies comparing helium-ion therapy and brachytherapy for medium-sized uveal melanomas found improved local control, anatomically eye preservation and disease-free survival with charged-particle therapy. Plaque brachytherapy can achieve similar outcomes with careful patient and tumor selection.16‒18
SRS has been under clinical investigation for the treatment of uveal melanoma at last decades. According to clinical experience the therapeutic single dose has been reduced to as low as 35.0Gy over the past fewyears without unfavorable reduction in tumor control.6 At the 50% isodose doses of 40.0Gy delivered result in a good local tumor control and acceptable toxicity. Fractionated stereotactic radiotherapy (SRT) has gained additional interest since radiobiological studies indicate a possible advantage of hypofractionated treatment over a single, very large fraction to sterilize uveal melanoma cell lines. Linear accelerators (LINAC) have the advantage of a feasible fractionation. The efficacy of SRS studies with a hypofractionated scheme (4-5 fractions) and total doses between 50.0 and 70.0Gy has been proven. Their results had good local tumor control rates over 90%. Stereotactic radiotherapy offers a non-invasive alternative to enucleating and brachytherapy in the management of juxtapapillary choroidal melanoma with a high tumor control rate.19‒21
The management of patients with uveal melanoma has changed towards globe sparing techniques radically in the last period. Therapeutical alternatives to the radical enucleating (or exenteration of the orbit) in advanced stage of uveal melanoma vary from observation to transpupillary thermotherapy in T1 stage uveal melanoma, block-excision, and endoresection with pars plana vitrectomy combined with brachytherapy using a variety of radioisotopes in T2 stage uveal melanoma, or in T1 to T3 stage uveal melanoma external beam radiotherapy, charged particles, Leksell gama knife and SRS can be used.22‒24
Proton beam radiotherapy of uveal melanoma by a 62MeV cyclotron can achieve high rates of local tumor control and good visual acuity outcome, but depends on tumor size and location. In 5year interval after therapy local control rates can exceed 95%.25
No multicentre trial to assess safety, dosimetry, and efficacy of SRS or to evaluate outcomes of gamma knife radiosurgery for melanoma has been performed yet. SRS can have similar good local tumor control rate, mortality rate, and metastasis rate in comparison with brachytherapy. SRS and gamma knife radiosurgery may be an suitable alternative therapy to treat uveal melanoma in patients, where conventional brachytherapy is not available.26,27
Stereotactic proton-beam therapy is another option for large tumors and may help spare the need for enucleating and vision loss. A large retrospective study of 492 patients with T3 to T4 choroidal melanomas demonstrated a 5year local control rate of 94% and a 19.5% enucleating rate that decreased over time.28
Secondary radiogenic side effects like radiation retinopathy, postradiation cataract, neuroretinopathy, maculopathy or secondary glaucoma are present also by other radiotherapy modalities. Secondary radiogenic side effects can cause the majority of secondary visual acuity loss and lead to secondary enucleatings. For intraocular uveal melanoma are effective treatment modalities with promising late tumor control and toxicity rates and mortality rates stereotactic photon beam radiotherapies (SRS and SRT). Today is not available a multi-centre trial to compare the outcomes following SRS with other irradiation methods in uveal melanoma. SRS of uveal melanoma, based on CT and MRI images, is a safe and precise treatment option. Local control was found to be excellent.5,29 LINAC based SRS for choroidal or uveal melanoma is well tolerated and can be offered to patients with medium sized but also unfavorably localized uveal melanoma.29
Only few large, prospective, randomized trials were designed to compare mortality figures for medium-sized melanomas treated by brachytherapy, enucleating or SRS.30‒32
SRS and gamma knife radio surgery may be an appropriate alternative for uveal melanoma patients’ therapy where tumors are ineligible for brachytherapy. One of the main goals of the “conservative treatment” like SRS is the anatomical eye globe retention. But in some cases enucleating must be necessary due to secondary complications, such as secondary glaucoma.7,33
In tumors localized near to optic disc can develop optic neuropathy following SRS. Stereotactic planning scheme carefully planned with the maximal dose to the optic nerve 8.0Gy can reduce the toxicity. In the past uveal melanoma has been considered a radioresistant tumor, but SRS is nowadays considered to be an alternative to treat uveal malignant melanoma. Single one-day sessions SRS with 35.0Gy according to our previous study outcomes is sufficient to treat small and middle stage melanoma.5,7
A non-invasive alternative in the treatment of uveal melanoma with a high tumor control is SRS. According to our previous studies one step LINAC based SRS with a single dose 35.0Gy with a mechanical immobilization of the eye globe with four sutures is a highly effective method to treat small and middle stage uveal melanoma.6,7
Important prognostic factors for death from metastatic melanoma include the size of the tumor (the larger the tumor, the worse the prognosis), the location of the tumor (tumors within the ciliary body are associated with a poorer prognosis than those confined to the choroid), the age of the patient at the time of diagnosis (the older the patient, the worse the short-term survival prognosis) and extrascleral tumor extension.34
Preservation of the eye globe and useful visual acuity in patients are the obvious advantages of conservative treatment with radiation compared with radical surgery - enucleating. However questions have been raised regarding the efficacy of this treatment in limiting metastatic spread of the tumor.26,32
Comparing irradiation with enucleating indicated no significant differences in the survival rates. Overall 5-year mortality rates were comparable in patients treated by enucleating and by plaque radio-therapy (iodine 125).30 Proton beam irradiation is highly successful in achieving local control of intraocular melanoma. Many patients maintain some degree of function in the eye for long periods after treatment. Overall rates of metastatic disease are comparable to those observed after enucleating; thus, enucleating should be limited to patients with large T3/T4 tumors in whom the eye is unlikely to be salvaged by irradiation.34 In our study the individual Kaplan-Meier plots show development that secondary enucleating after SRS leads to lower the risk of death for enucleated patients.
According to our results one-day session SRS with 35.0Gy is a successful treatment of small and middle stage uveal melanoma. Survival rates in 5year interval and necessity of secondary enucleating due to complications after one day sessions linear accelerator irradiation are both comparable to other techniques. Importantly, the overall survival of patients who underwent secondary enucleating was not different from the survival of patients without enucleating. The development of optic neuropathy is presumably associated with higher irradiation of the optic disc and there is significant association of glaucoma with the presence of optic neuropathy.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Financial support: no financial support received. Payment of Article Processing Charge (APC) supported by KEGA 016UK-4/2018.
The statistic data used to support the findings of this study are included within the article.

©2018 Furdova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.