Advances in
eISSN: 2377-4290


Research Article Volume 9 Issue 2
11Specialty Registrar, University College London, UK
2Moorfields Eye Hospital NHS Foundation Trust, UK
Correspondence: Shahab Shahipasand, Specialty Registrar, University College London, UK, Moorfields Eye Hospital 162 City Road, London, EC1V 2PD, Tel +447500968712, Tel +447500968712
Received: February 28, 2019 | Published: March 26, 2019
Citation: Shahipasand S, Mohammed-Noriega J, Sim D, et al. Nailfold capillaroscopy with a commercially available optical coherence tomography angiography for ophthalmic use. Adv Ophthalmol Vis Syst. 2019;9(2):38-42. DOI: 10.15406/aovs.2019.09.00343
Visualising capillaries in the retina and the nailfold provides an opportunity to improve our understanding of various systemic disorders including cardiovascular, metabolic, rheumatic, and eye diseases.1 –5 Traditional nailfold capillaroscopy has been primarily utilised, during the last 40 years, to study the vascular components of connective tissue diseases.6,7 It has been established as a safe and non-invasive diagnostic tool that allows high magnification view of skin microcirculation as well assessment of capillary density and blood flow velocity.8,9 In ophthalmology, a number of studies have been conducted to assess eye diseases and their relationship with nailfold changes since 1980s.6,10 A recent example is the association found between nailfold microhaemorrhages or loss of nail capillaries and glaucoma5 and in exfoliation syndrome.11 Capillaroscopy findings have been also associated with diabetic retinopathy and central serous chorioretinopathy.12,13
Optical Coherence Tomography (OCT) has had a revolutionary impact in evaluation of anatomical structures of the eye and became an important part of everyday clinical practice and research14 Technological advances have enhanced various aspects of OCT images including scanning speed, resolution and measurement of vascular flow.9 Optical Coherence Tomography Angiography (OCTA) is an imaging modality that enables blood vessels to be visualised via motion contrast imaging of red blood cells moving across sequential B scans at a given cross-section.6 Originally designed for the retina, OCTA is also being established as a useful non-invasive tool in imaging cornea and anterior segment of the eye.15
Traditional nailfold capillaroscopy has limitations in acquiring images from highly pigmented skin [16]12 and the need of skilled user to perform and interpret the test. New imaging modalities have been described in the literature and a modification of OCTA - optical micro-angiography (OMAG) - was described as a technique to provide a three-dimensional blood perfusion map using Fourier Domain OCT (FD-OCT).17,18 In dermatology, the Dynamic-OCT has shown good ability to image different skin disease and its vascular component.19–21 However, this is currently limited by relatively long scanning time and is yet to be commercially available. We propose a pilot study to evaluate whether commercially available OCTA designed for ophthalmological purpose can visualise the nailfold capillaries. The use of the same instrument that is already available in eye clinics could provide an easy platform to introduce the use of nailfold capillaroscopy in ophthalmology and conduct further studies in nailfold changes and systemic and eye disorders using the same instrument.
We conducted a cross-sectional observational study on ten healthy volunteers with no previous history of ophthalmic or rheumatic disease that attended Moorfields Eye Hospital from 1 February 2017 to 30 April 2017 only for the purpose of this research. A trained operator performed OCTA scans using the AngioVue system (Optovue, Fremont, California, USA), which utilises the split-spectrum amplitude decorrelation angiography algorithm.22 The scans were obtained via AngioRetina mode (intended for imaging of retina), but with the addition of the anterior segment optical adaptor lens (intended for imaging the cornea and anterior segment of the eye).15 Axial resolution and beam width of each scan were 5μm and 22μm respectively, with a light source centred on 840nm. Consecutive B scans consisting of 304 x 304 A-scans at 70,000 scans per second are captured in a transverse direction. The instrument constructs a 3-D scan cube in approximately four seconds.23 The OCT signal position and quality were optimised using the “Auto F” and “Auto Z” to find the best focus to visualise the nailfold. Auto P was set to zero to avoid any correction in polarization that would be unnecessary. Scans were repeated if motion or initial position of the finger affected the quality of the images.
All participants were recommended to avoid smoking or drinking caffeine for at least four hours prior to the scan. They were also asked not to remove fingernail cuticle four weeks prior to the scan and avoid trauma to fingers. The participants were seated in room temperature for approximately 20-30 minutes before the scan. We used the fourth digit of the non-dominant hand in the subjects to obtain OCTA scans (3x3 mm volume cubes). The subjects were asked to rest their finger on the lens without pressing during scanning (Figure 1).

Figure 1 Angio vue System with anterior segment adapter and 4th finger in position for capillaroscopy.
We invited a participant to a second visit to perform a traditional nailfold capillaroscopy with Optilia Digital Capillaroscope (Optilia Instruments AB, Sollentuna Sweden) at 200X magnification. We followed the same examination conditions as with OCTA, and imaged the same digit. We applied natural almond oil to the nailfold to improve vessel visualization. The images were exported from OptiPix Lite software and compare side by side with the corresponding OCTA scan.
The OCT data was further analysed and three-dimensional reconstructions were created. Data were exported in RAW format and split using an executable file provided by Optovue. The RAW volumes were imported in Matlab and exported as tiff stacks. The stacks were then imported in Image J. After contrast adjustment of the OCT angiography images, the structural and angiography volumes were combined to form multichannel images. The 3D Volume Viewer plug in for ImageJ was used to visualize the combined volumes.
The skin colour of the participants was assessed according to the Fitzpatrick classification[24]. In this classification, three factors are addressed: 1) genetic disposition and overall appearance including skin colour, hair colour and eye colour; 2) Reaction to sun exposure and 3) tanning habits. The scores for each category are cumulatively added to give a final score out of 40. Table 1 shows classification of skin types using the Fitzpatrick scale.
Fitzpatrick scale |
Description |
Type I |
Always burns, never tans (pale white skin) |
Type II |
Usually burns, then tans (white skin) |
Type III |
May burn, tans well (cream/white brown skin) |
Type IV |
Rarely burns, tans well (moderate brown skin) |
Type V |
Very rarely burns, tans well, (dark brown to black skin) |
Table 1 Fitzpatrick scale
In this study, we performed OCTA scans in 10 healthy participants (6 men, 4 women, mean age 28.2 +/- 6.29 Table 2 shows demographic information of the subjects including age, sex and skin type. We scanned two subjects with deep brown skin, 3 subjects with white skin and 5 subjects ranging from light brown to moderately brown skin. Nailfold capillaries were successfully detectable in 100% of the OCTA scans. Figure 2 is an example of the OCTA scan obtained from subject 7 along with an image of the nailfold of the same digit using the traditional method for nailfold capillaroscopy. We measured the flow area and constructed a vessel density map using the built-in software of the instrument (RTVue XR, version 2016.2.0.35; Optovue Inc). Furthermore, we were able to obtain an automatic measurement of vessel density and flow area per individual scan as elucidated in Figure 3. Three-dimensional reconstructions were created and modified to allow an easy identification of the vessels and the surrounding anatomical structures. The front row of capillaries is easily identified in relation to the nail and the surface of the skin (Figure 4).
Subject |
Sex |
Age |
Fitzpatrick |
1 |
M |
21 |
IV |
2 |
M |
25 |
I |
3 |
M |
30 |
III |
4 |
M |
43 |
II |
5 |
M |
25 |
III |
6 |
M |
24 |
V |
7 |
F |
28 |
IV |
8 |
F |
26 |
V |
9 |
F |
26 |
IV |
10 |
F |
34 |
II |
Table 2 Demographic and skin characteristics of participants
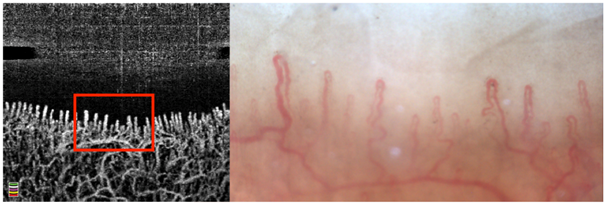
Figure 2 Nailfold capillaroscopy of the same finger of participant number 7. (Left) Using Optical Coherence Tomography Angiography and (Right) traditional capillaroscopy. The area delineated in red is the same area that was scanned with traditional capillaroscopy.

Figure 3 Optovue printout using the Angio Retina scan for nailfold capillaroscopy. (A) Area in mm2 manually delineated and automatically segmented for vessel area and flow area. (B, C, D) en face, longitudinal and axial cuts with red and green lines marking the corresponding location of the selected vessel. (E) Automatic measure of vessel density and thickness with colour coded map.

Figure 4 Three-dimensional reconstructions from Raw OCT structural and angiographic data. (Left) Coronal view depicting the first row of capillaries. (Right) sagittal view at the level of line at left image demonstrating the relationship between the front row of capillaries and the nail. Arrows point at the front row of capillaries.
We proved for the first time that a commercially available OCT-A for ophthalmology can identify some of the morphological characteristics typically assess during conventional nailfold capillaroscopy. The recent interest in nailfold capillaroscopy anomalies and eye diseases such as glaucoma, diabetic retinopathy and central serous chorioretinopathy are good examples of the growing interest in assessing other microcirculations in addition to the retina to improve our understanding of the aetiology and pathophysiology of these conditions. There is no doubt that OCTA can provide valuable clinical information and in depth understanding of various ophthalmic disorders and it would be desirable to be able to use the same instrument when conducting research to evaluate relationships between systemic microcirculatory findings and diseases of the eye. To our knowledge, commercially available OCTA has not been reported as an alternative modality for assessment of nailfold microcirculation at the time of this publication.
OCTA offers additional benefits over the traditional nailfold capillaroscopy technique. Firstly, in traditional capillaroscopy, the strong absorption of light in the visible spectrum by melanin affects the findings of individuals with deeply pigmented skin.16 Quality of the OCTA scans of subjects with darker skin types (IV and V) did not differ significantly from individuals with lighter skin shades. Secondly, traditional capillaroscopy does not provide a good view of capillaries when they overlap in different dephts, while OCTA allows for appreciation of the depth of vessels (Figures 3&4) and enables a more comprehensive outlook of the microvasculature under the skin. Capillary density (i.e. the number of capillaries per unit area of skin) are defined by two methods for nailfold in the literature: a) the number of perfused capillaries per square millimetre or b) the number of capillaries in a one millimetre span of the distal row.25 In both methods, capillary density is typically obtained by counting of the visible capillary loops. Both density methods are reproducible using the OCTA scans and can be done automatically. Although recently capillary counting algorithms have been proposed for use in traditional nailfold capillaroscopy,26 the built-in software of OCTA not only allows rapid and automatic quantification of capillary density but also offer an estimation of the blood flow. Another advantage of OCTA is the rapid imaging speed. Each image takes approximately 15 seconds to be obtained including the time lapsed for correction of position, focus and scanning time. Currently, the skin needs to be placed in contact with the anterior segment adapter lens for optimal scan quality. However, application of immersion oil is not required unlike capillaroscopy.27 Finally operator dependency of OCTA is advantageous over that of traditional capillaroscopy that requires the operator to maintain the correct position and pressure of the probe over the skin. This has resulted in previous reports of quantification and classification of microcirculatory abnormalities being affected by wide operator dependence in the traditional technique.28 OCTA images can be obtained once the user is able to adjust the position of nailfold correctly in relation to the lens and is familiar with the software parameters. No specific training is therefore required. An important limitation is the use of an adaptor lens designed to scan the anterior segment of the eye and software intended to automatically segment retinal images. The previous limitations affected the quality of some images and not all the nailfold scans had enough quality to perform quantitative analysis. Currently, it is not possible to visualise microhaemorrhages in the nailfold with this technique but this might be overcome in the future as multiple imaging modalities are used simultaneously to strength the overall ability of the instruments. Another disadvantage of OCTA in comparison with the traditional method is the magnification power. The current magnification of OCTA is design for ophthalmic use with only two or three different options while optical probes for traditional capillaroscopy range from 50x to 1000x. Finally, the median age of the participants was 26 (range 21-43). It would, therefore, be appropriate to select a broader age range for future studies and include patients with pathology to assess the ability of OCTA to identify pathological changes in capillaries.
The last study looking at outcomes for nursing and medical staff was performed over a decade ago.7 Conservative treatment was found to be successful for 29% of cysts while surgical treatment was successful for 72%. There was no significant difference in treatment outcome between nurse and SHO groups.7 Our study showed mirrors results with patients overall rating the service to be good or excellent. Our study concentrates on assessing the quality of care patients received during the whole process. All patients found nurse treatment acceptable with a high level of patient satisfaction, adequate pain control and quality of care. This was similar for the medical staff. Most comments were regards to general practitioner referrals. Questionnaires were given on the day of surgery to ensure compliance and this may have introduced bias in the results. Staff performing the procedure was aware of the survey which may have bias the high satisfaction scores. Patients may have felt they had to give a higher overall score. Although all patients who had the surgery were given questionnaires they were entirely voluntary. Patients were required to hand the survey to the reception staff or allocated box at reception. It is possible a cohort of patients was missed and did not hand in the questionnaire. The questionnaires collected in a sealed box were only handled by the investigator who did not perform any of the procedures. At presents approximately 7 chalazion surgeries are performed on each minor operations list. Our modest numbers reflects list cancellations during leave and the Christmas period. As more nursing staff are trained to perform chalazion surgery cancelled lists may not be an issue with adequate cross cover of medical and nursing staff. Patient also failed to turn up and this may be due to miss information from the general practice referral. This study further reiterates the need to address patient quality of care in an under resourced health system. Nurse led chalazion removal has shown to a safe effective solution to future economic rationalization for elective minor procedures. We have shown a high level of quality of care for the service and scope for future cost effect growth.
In summary, we describe a proof-of-concept study of a commercially available OCTA ophthalmic system, potentially useful for evaluation of nailfold capillaroscopy. Our study proves that the prospects of this non-invasive, rapid modality for broader applications in microvascular research are promising. Future studies are desirable to quantitatively assess whether this technique is suitable for evaluation of nailfold capillaries compared with other available techniques.
None.
Author declares there are no conflicts of interest towards this article.

©2019 Shahipasand, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.