Advances in
eISSN: 2377-4290


Research Article Volume 12 Issue 3
1Jerabek-Zabalo Rio Cuarto Ophthalmological Network, Córdoba, Argentina
2Nueva Visión Rio Cuarto Private Eye Clinic, Córdoba, Argentina
Correspondence: Ana Silvina Nasif Maida, Ophthalmology Network, Córdoba, Argentina, Belgrano 417b-New Vision Rio Cuarto Private Eye Clinic, Córdoba, Argentina
Received: November 04, 2022 | Published: November 16, 2022
Citation: Maida ASN, Varas S, Jerabek RV. Krukenberg spindle and its implications. Adv Ophthalmol Vis Syst. 2022;12(3):81-85. DOI: 10.15406/aovs.2022.12.00425
Pigment dispersion syndrome (PDS) and pigmentary glaucoma (PG) constitute a spectrum of diseases characterized by excessive release of pigment from the iris. PDS is typically bilateral and most often affects young, myopic men. The classic findings, “Classic Triad”, of the anterior segment include pigment deposition in the corneal endothelium (Krukenberg spindle), dense pigmentation of the trabecular meshwork (TM) and transillumination defects of the iris in the midperiphery.1,2 A characteristic concave iris configuration has also been described to facilitate pigment release through increased iridozonular contact.3–5 Over time, the chronic release of pigment can lead to an increase in intraocular pressure, which can evolve into a PG. The etiology is congenital, one theory is that there is congenital mesodermal dysgenesis or degeneration of the primary iris, although it is thought that the best theory is an affection of the middle third of the eye during the third trimester, which could explain the association with retinal conditions such as lattice degeneration, retinal tears, and retinal detachment (RD). The incidence of DR in patients with PDS/GP is as high as 12% Vs normal population 0.001%. The rate or risk of progression to GP among patients with PDS has been reported to be 10% at 5 years and 15% at 15 years in a prospective trial showing that intraocular pressure (IOP) > 21 mmHg at presentation is a key risk factor for the progression of this disease.
In the following work, a clinical case of a 32-year-old patient with PDS is presented. At the time of consultation, he only reported having had episodes of sporadic intense headaches accompanied by blurred vision. It was decided to carry out an exhaustive study to rule out different differential diagnoses as well as that he was not undergoing a PG, and to determine the need to perform a prophylactic laser peripheral iridotomy (LPI).
A 32-year-old male patient presented for a routine ophthalmological check-up. He reported episodes of sporadic intense headaches accompanied by blurred vision. He denies pathological personal history (PPA) and ophthalmological personal history (APO).
On examination, visual acuity without optical correction was 10/10 AO.
Refraction right eye (RE) +0.25 -0.25x25° left eye (LE) +0.25 -0.50X35°.
Biomicroscopy (BMC) showed a spindle-shaped pigment deposit in the bilateral corneal endothelium (Krukenberg's spindle).
Figure 1 Intraocular pressure (IOP) Goldmann tonometry (11 am) RE 17 mmHg LE 15 mmHg.
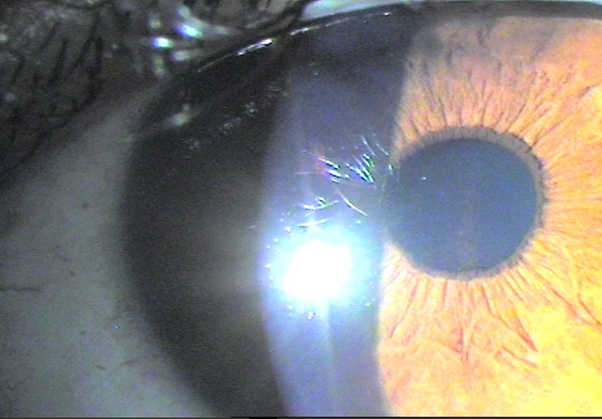
Figure 1 Pigmentary deposit is observed at the level of the corneal endothelium arranged vertically or in a spindle (Krukenberg spindle) in BE.
Possible differential diagnoses that could occur with pigment deposit in the corneal endothelium (Krukenberg spindle) were considered; 1.2
Gonioscopy: in the iridocorneal angle, angular hyperpigmentation was observed at the level of the bilateral trabeculum, homogeneous and occupying 360°.
Intraocular pressure curve
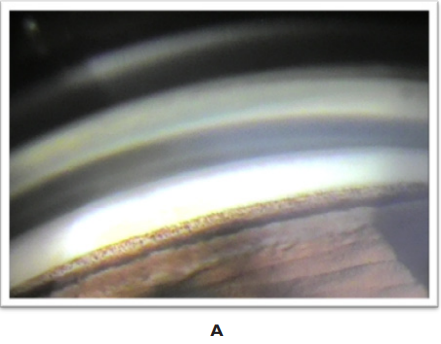
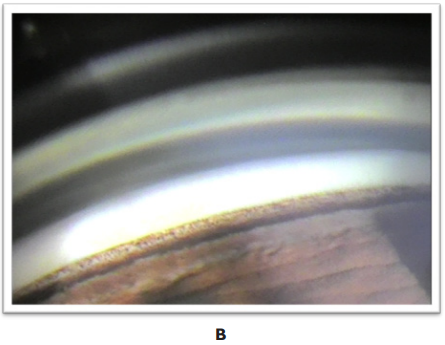
Figure 2 Iridocorneal angle: hyperpigmentation of the bilateral, homogeneous trabecular meshwork is observed, occupying 360°.
6am: DO: 19mmHg
LE: 17 mmHg
8am: DO: 18mmHg
LE: 16 mmHg
11am: OD: 17mmHg
LE: 16 mmHg
17pm: DO: 16mmHg
LE: 14 mmHg
Pachymetric correction
DO: 21mmHg
LE: 18 mmHg
Pachymetry:
In the eye fundus, pink papillae with clear edges were observed, slight asymmetry of papillary excavation, RE 2/6 and LE 3/6, macula s/p, periphery, no predisposing lesions for DR were observed in BE.
Due to the papillary cup asymmetry, it was decided to perform optical coherence tomography (OCT) of the papillae, where a normality of the nerve fiber layer (NFL) in BE was confirmed (Figure 3–8).
Pigment dispersion syndrome (PDS) can be associated with ocular hypertension (OH) or glaucoma (GP) and is usually bilateral. Pigmented ocular hypertension (HOP): It is defined as PDS with elevated IOP and without glaucomatous optic neuropathy.
Pigmentary glaucoma (GP): It is defined as a PDS with a glaucomatous optic neuropathy.
The results of the studies carried out on the patient made it possible to rule out PHTH and PG, since he had IOP < 21 mmHg in the daily curve and did not present optic neuropathy or CVC alterations.7
Anterior uveitis can cause pigmented epitheliitis of the iris with pigment release and deposition in ocular structures. A post-tubeitis pigment dispersion was ruled out as there was no positive APO, and no pupillary alterations or anterior and/or posterior and angular synechiae were found on examination.7–9 Intraocular surgery, in particular, lenses fixed in the sulcus (closer to the iris) or trauma (blunt or penetrating) may present features similar to SSD, with defects in transillumination of the iris, pigmented cells in the anterior chamber, increased pigmentation in MT and elevated IOP. However, the patient did not present APO from ocular trauma or intraocular surgeries.1,7,8,10,11
Pseudoexfoliation syndrome may be associated with iris translumination defects, increased pigmentation of the patchy type TM by 50%, usually unilateral, and elevated IOP. However, the patient did not present deposits of pseudoexfoliation, nor transillumination defects of the iris and the angular pigment dispersion was homogeneous and occupied 360° and was bilateral.7,12 The presence of a pigmented intraocular tumor was also ruled out by gonioscopy and UBM, where it was also possible to appreciate a posterior concavity of the iris and an intimate iridozonular contact.1,7,9
Therefore, we can deduce that the patient presented PPPS, whose classic triad is the presence of pigment in the corneal endothelium (Krukenberg spindle), increased pigmentation of the homogeneous type TM and occupying 360° in AO and transillumination of the iris in mid-periphery.1–3 However, he did not present the latter sign, but recent studies updating the signs of PDS and GP carried out by Kingsley show that some people with highly pigmented irises may have difficulty visualizing this sign. In terms of epidemiology, young patients (30-50 years), Caucasian and myopic suffer more from it.1–3 Although the patient was emmetropic, this study also shows us that 12-42% of people with this disease may be emmetropic (-1D / +1D). A doubtful symptom of this disease was the presence of transient sporadic intense headaches accompanied by blurred vision, but the patient denied that their appearance was after physical exercise. All studies report that it occurs after it, Haynes explains that there is a large release of pigment due to an increase in iridozonular friction, corneal edema and very high IOP that would justify the symptoms and diurnal fluctuations in IOP of these patients with compared to other types of glaucoma.17,18
The treatment (TTO) of PDS and GP depends on the classification of Richter et al. Defined in several stages: (3.19)
Pigment dispersion was defined as active when there is a:
If pigment dispersion is inactive and IOP is stable, TTO is expectant and IOP controls should be performed using pressure curves and detection of changes that indicate active pigment dispersion. If, on the other hand, there is active pigment dispersion with stable IOP and the presence of inverse pupillary block (iridian concavity), expectant TTO can be performed or, highly controversial, the use of pilocarpine or peripheral laser iridotomy (IPL). Pilocarpine is the ideal therapy, but its use is highly controversial since it lowers IOP, reduces iridozonular friction through pupillary constriction and increases aqueous humor outflow, but it can produce accommodative spasm; increases the risk of DR and can lead to cataract formation. Kurwa was the first to report the successful use of IPL as a therapy for SSD and GP in 1984.13,14 However, iridotomy was not widely accepted as a treatment because no pathophysiological explanation could be offered. It was not until 1991 that IPL was popularized by Campbell.15 when he observed that the effect it produced was a flattening of the iris. Campbell's5 and Karickhoff's20 concept of posterior iris concavity and reverse pupillary block as the cause of pigment dispersion provided the explanation for the IPL treatment effect. IPL equalizes the pressure between the anterior and posterior chambers, relieving reverse pupillary block, flattening the iris, and reversing the posterior tilt of the iris.19–22 Figure 9 such that prevents further release of pigment.23
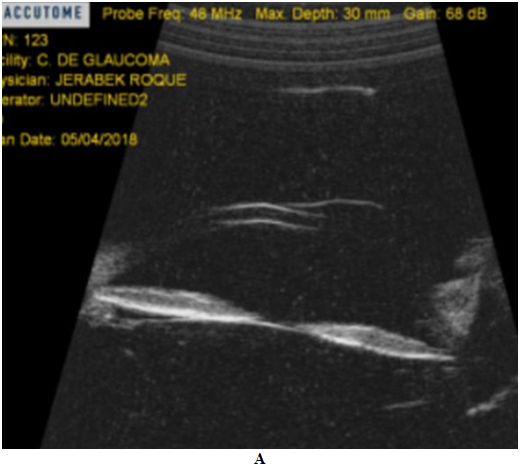

Figure 9 Ultrabiomicroscopy shows posterior concavity of the iris and greater iridozonular contact in BE, rest of the structures S/P.
Gandolfi and Vecchi24 have shown that IPL prevented long-term increases in IOP in patients with PDS. In their study, only 5% of eyes randomized to IPL treatment had an increase in IOP greater than 5 mmHg, compared to 52% of the control group, over a 2-year period. The advantageous effect of iridotomy was more significant in patients <40 years of age, probably because the disease is in an active phase in younger patients. An IPL alone is not the ideal treatment for PDS/GP, unfortunately it is not successful in all cases. No benefit in preventing or reducing long-term CVC progression was seen in SSD and GP.25,26 There is no scientific evidence, to date, to perform IPL as a treatment in patients with PDS or GP, since the effect of IPL is likely to be useful only in active stages of the disease and if iridian concavity is present. Significant later IPL is unlikely to be of benefit in eyes that already have permanent trabecular damage and/or progressive Glaucoma because it does not lower IOP.27 Therefore, IPL is likely to be beneficial before the development of GP, offering patients with PDS an appropriate prophylactic procedure. However, performing this procedure in a patient without overt disease remains controversial, particularly because IPL generates pigment dispersion that can further damage the MT and/or cause elevated IOP spikes as an adverse or secondary effect.
It was concluded, therefore, that the patient presented PPPS in the inactive phase. In this case, we must control the possible appearance of the active pigment dispersion stage and the IOP figures by means of pressure curves to determine when to perform an IPL as a prophylactic treatment.28,29 The ideal eye to be treated with IPL would be one that presents PDS with a posterior iridian concavity and an increase in pigment dispersion (active phase) or an increase in IOP with exercise or pupillary dilation with phenylephrine that is corroborated in the office.30 Carry out a follow-up through complementary studies for the early detection of alterations in the optic nerve and/or CVC.
None.
The authors declare no conflicts of interest.

©2022 Maida, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.