Advances in
eISSN: 2377-4290


Research Article Volume 2 Issue 4
Ophthalmologist Yazan Zahran eye clinic Jordan
Correspondence: Yazan zahran Ophthalmologist Yazan Zahran eye clinic 9 Suliman adeedi street Amman Po Box 143539 Jordan, Tel 962790573036
Received: September 27, 2014 | Published: June 8, 2015
Citation: Zahran Y. How to read fluorescein angiography. Adv Ophthalmol Vis Syst. 2015;2(4):131-136. DOI: 10.15406/aovs.2015.02.00054
How to Read Fluorescein Angiography
Keywords:fluorescein angiography, retina, uvea, choroid
RPE, retinal pigment epithelium; BRB, blood-retina barrier; FAZ, foveal avascular zone
Before we discuss the fluorescein angiography in details we must give brief anatomy of the retina and choroid. The retina composed of two parts: neurosensory retina which composed of 9 layers the internal limiting membrane, the nerve fiber layer, the ganglion cell layer, the inner plexiform layer, the inner nuclear layer, the outer plexiform layer, the outer nuclear layer, the rod and cone inner and outer segments, the external limiting membrane (Figure 1). Retinal pigment epithelium (RPE) which composed from monolayer of cells & its functions are: Absorption of scattered light, Control of fluid and nutrients in the subretinal space (blood retinal Barrier function), visual pigment regeneration and synthesis.1 Synthesis of growth factors to modulate adjacent structures maintenance of retinal adhesion phagocytosis and digestion of photoreceptor wastes electrical homeostasis. Regeneration and repair after injury or surgery.
Blood supply
The retina receives its nutrition from two discrete circulatory systems-the retinal blood vessels and the uveal or choroidal blood vessels both are derived from the ophthalmic artery, which is the first branch of the internal carotid artery melanocytes, and connective tissue From anterior to posterior, the uveal tract has three distinct subdivisions the iris, the ciliary body, and the choroid the iris and ciliary body are referred to as the anterior uvea. The posterior uvea is synonymous with the choroids. The choroid can be subdivided into three distinct parts from internal to external Bruch’s Membrane choriocapillaris; and the vessel layer. The choroid, located between the retina and the Sclera (Figure 2).
Function
The choroid nourishes the outer retina and a portion of the optic nerve.
Blood supply
Mainly from posterior ciliary arteries. The ocular fundus has two separate vascular systems-retinal and choroidal-separated by a specialized pigmented monolayer the retinal pigment epithelium (RPE) (Figure 3) (Figure 4). The choroid and it’s vasculature lie posterior to the RPE, the fluorescein angiographic patterns of the posterior uvea are, therefore, always partially obscured by the RPE.2 The degree of pigmentation and the pathologic changes in this pigmented layer markedly influence the choroidal angiographic appearance there is two barriers in the retina: 1. Outer retinal barrier: consists of tight junctions between the retinal pigment epithelium (RPE) 2. Inner retinal barrier: consists of non-fenestrated capillaries of retinal circulation (Figure 5). These barriers called the blood-retina barrier (BRB). It’s main function is control and prevents entry of certain substance to the tissue of the retina.
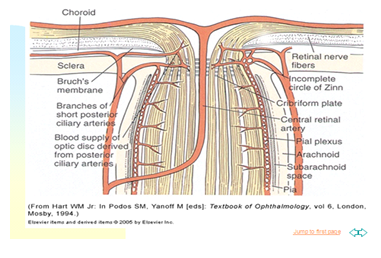
Figure 3 The ocular fundus has two separate vascular systems-retinal and choroidal-separated by a specialized pigmented monolayer the retinal pigment epithelium.
Any disturbance in this tissue and their vasculature will lead to pathological change and functional disturbance of the vision. The development of FA increased the understanding of retinal and choroidal pathology and has become the standard both in the literature and in clinical practice to diagnose and guide the treatment of the most common retinal diseases encountered in ophthalmology.
Methods and materials
The sodium fluorescein molecule has fluorescence as part of it characters which means-its ability to absorb a photon of light of shorter wavelength and emit a photon of light of a longer wavelength. Sodium fluorescein is orange in color, it’s molecular weight of 376. Approximately 80% of fluorescein dye remains in the intravascular compartment binding to albumin, while remaining is free. The dye is metabolized by both hepatic and renal pathways and is excreted in the urine within 24-36 hours.
Technique
The patient is seated comfortably in front of the fundus camera, and an intravenous cannula inserted a standard venous cannula should be used rather than a less secure ‘butterfly’ winged infusion set. After cannulation, the line should be flushed with normal saline to check patency and exclude extravasation, fluorescein, usually 5 mL of a 10% solution, is drawn up into a syringe in eyes with opaque media, 3 ml of 25% solution may afford better results If not already obtained, colour photographs are taken. A ‘red-free’ image is captured. If indicated, a pre-injection study is performed to detect autofluorescence (see below), with both the excitation and barrier filters in place.
Fluorescein is injected over the course of a few seconds. Images are taken at approximately 1 second intervals, beginning 5-10 seconds after injection and continuing through the desired phases If the pathology is monocular, control pictures of the opposite eye should still be taken, usually after the transit phase has been photographed in one eye If appropriate, late photographs may be taken after 10 minutes to show leakage, and occasionally after 20 minutes.3 Stereo images may be helpful to demonstrate elevation, and are usually taken by manually repositioning the camera sideways or by using a special device (a stereo separator) to adjust the image; such images are actually pseudo stereo, true stereo requiring simultaneous pictures from differing angles.
Normal angiographic pattern
Consists of six stages we will discuss them in details:
Choroidal phase: (Figure 6) begins 10 to 12 seconds after dye injection in young and 12 to 15 seconds after injection in older patients, early choroidal fluorescence is faint patchy and irregular called the choroidal flush. Areas of choroidal filling and non-filling become more distinct called patchy choroidal filling.
Arterial phase: (Figure 7) Starts 1 to 3 seconds after choroidal fluorescence with filling of the central retinal artery after the central retinal artery begins to fill, the dye flows into the retinal arterioles, pre capillary arterioles, the capillaries, the post capillary venules, and finally the retinal veins.
Early arteriovenous phase: (Figure 8) The fluorescein dye from the smaller venules enters the vein along their walls resulting in a laminar flow of the dye in the vein. As the vascular flow is faster in the center of the vessel than on its side, the fluorescein dye sticks to the walls of the vein: Another contributing factor for laminar flow. With time the laminae along the walls of the veins become thicker.
Arteriovenous phase: (Figure 9) The dye completely fills the lumen of the vein. Perifoveal capillary network is best visualized at 20 to 25 seconds after the injection of the dye when the concentration of the dye is maximum The fovea appears hypo fluorescent because of the absence of the blood vessels in the foveal avascular zone (FAZ) and due to the blockage of the background choroidal fluorescence by the increased pigment in the tall RPE cells at the fovea (Figure 10).
Recirculation phase & late phase: (Figure 11) Recirculation phase: Begins about 30 seconds after the dye injection, fluorescence within the vessels reduces as lower concentration of fluorescein recirculates Late phase: Retinal vessels are empty of the fluorescein dye by 10 minutes after injection, disc remains hyper fluorescent in late films due to staining .
The abnormalities of FA image is divided into two main patterns the Hyper Fluorescence or the hypo fluorescence Specifying the pattern plus following it change with time will let you define easily the diagnostic fluorescein pattern let’s start with the hypo fluorescence.4
Hypo fluorescence
Is mainly divided into 1) blocked fluorescence and 2) vascular filling defect. Blocked fluorescence: Blocked fluorescence when stimulation or visualization fluorescein blocked by Blood. (Figure 12) (Figure 13) Pigment (Figure 14) Fibrosis vascular filling defect: (Figure 15) Vascular filling defect: Occurs when the retinal or choroidal vessels do not fill properly as in non-perfusion of artery vein. Capillary hyper fluorescence is mainly divided into 1) Autofluorescence 2) Transmission defect (window defect) 3) Leaking 4) Pooling and 5) Staining. Auto fluorescence: (Figure 16) compounds absorb blue light and emit yellow-green light in a similar fashion to fluorescein. It is imaged much more effectively by scanning laser ophthalmoscopy but can also be detected on standard fundus photography in exposed optic nerve head drusen and sometimes with Lipofuscin in retinal drusen and other abnormalities such as astrocytic hamartoma and angioid streaks. Transmission defect (window defect): (Figure 17) A window defect refers to the choroidal fluorescence produced by a relative decrease or absence of pigment in the RPE or an absence of RPE. The hyper fluorescence occurs early and reaches its greatest intensity with the peak of choroidal filling leaking: (Figure 18) Leakage of fluorescein dye is defined as hyper fluorescence of fluorescein in the extra vascular space. Typically the area of fluorescence increases in both size and intensity as the study progresses.
The borders of hyper fluorescence become increasingly blurred, & the greatest intensity of the hyper fluorescence is appreciated in the late phases of the study pooling: (Figure 19) (Figure 20) Pooling refers to the accumulation of fluorescein dye into an anatomical space. Pooling is seen in both neural retina and RPE detachments the margins of the space trapping the fluorescein are usually distinct. Staining: (Figure 21) Staining results from fluorescein entry into a solid tissue such as a scar, optic nerve tissue, or sclera the pattern of hyper fluorescence with gradually increasing intensity of fluorescence, but the borders of the hyper fluorescence remain fixed throughout the angiogram process.
How to read FA?
None.
Author declares that there is no conflict of interest.

©2015 Zahran. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.