Advances in
eISSN: 2377-4290


Research Article Volume 1 Issue 3
1Department of Pathology of Eyes in Children, Russia
2Helmholtz Research Institute of Eye Diseases, Russia
Correspondence: Dmitry Ryabtsev, Department of Pathology of Eyes in Children, Moscow Helmholtz Research Institute of Eye Diseases, Sadovaya-Chernogryazskaya 14/19, Moscow, Russia, 105062
Received: October 26, 2014 | Published: November 25, 2014
Citation: Katargina L, Ryabtsev D. ERG and OCT changes incicatricial retinopathy of prematurity. Adv Ophthalmol Vis Syst. 2014;1(3):84-89. DOI: 10.15406/aovs.2014.01.00019
Purpose: to study changes of ERG and depth of retinal vessels bedding in children with cicatricial ROP.
Methods: We examined 58 children (82 eyes) aged from 6 months to 16 years old with cicatricial ROP. We evaluated maximal and flicker ERG, glial and AB-indexes and location of vessels in the retina using OCT.
Results: In GCL retinal vessels were only in 12 of 82 eyes (15%). In 29 eyes (35%)-they were located in NFL, and in 41 eyes (50%)-extraretinal (on the surface of the retina). There was a relation between the degree of displacement of retinal vessels and presence of extraretinal tissue, as well as a tendency to thinning of the retina in cases with extraretinal localization of the vessels (p <0.01). The incidence of traction retinal detachments and retinoschisis increases with growing degree of the displacement vessels. There was no statistically significant differences in the standard amplitude-time parameters of the ERG, but there was a tendency to growth of glial and AB-indexes with the increasing degree of displacement of the vascular bedding, which corresponds to the nature and frequency of late complications in cicatricial ROP.
Conclusion: These parameters can be important criteria of the severity of the process and important tests helping to detect a preclinical negative trend in cicatricial ROP.
Keywords: retinopathy of prematurity, spectral-domain, optical coherence tomography, retinal vessels, glial index, extraretinal growth, later complications
Retinopathy of prematurity (ROP) is a vascular proliferative retinal disease of premature infants and is one of the leading cause of childhood blindness and visual impairment1 and remains the focus of ophthalmologists around the world for decades. Pathological vascularization and subsequent proliferation play a major role in the development and progression of ROP. Criteria of diagnosis, prognosis and indications for treatment of ROP are based on the assessment of retinal vessels.2−5 Electrophysiology is very important in the assessment of the visual sensory system in ROP, considering the complex nature of the formation of vision. The degree of visual loss does not always correlate with the severity of pathology of the eye with ROP, and the level and extent of lesions of the visual sensory system with ROP can be estimated in detail only by electrophysiology.
Changes of the standard electroretinogram (ERG)-the maximum, scotopic, photopic, flicker (FERG), at different stages of ROP are well described by many authors.6–9 Glial index (b-wave/FERG 12 Hz), described by Zueva MV and Tsapenko IV in 1992-2009, reflects the state of glial cells in various degenerative and proliferative retinal conditions such as AMD, diabetic retinopathy, retinal detachment10−12 but it has not been studied well at ROP. Considering rather large avascular zones or coagulates in the periphery of the retina that could significantly affect the results of the study, using FERG at 12 Hz for the calculation of the glial index in patients with cicatricial ROP is not appropriate in our opinion. 30 Hz FERG, in contrast, is more stable and easy to use, because requires no changes to the standard examination protocol. AB-index (also called b/a ratio) was suggested by I. Perlman as an additional method in assessment of the retina.13 It is based on physiological considerations regarding the origin of the ERG components. In children with ROP this method can be used to assess retinal blood flow by evaluating the difference in function of outer and inner retina. At the present stage, despite the active introduction of morphometric techniques in ophthalmic practice only individual papers are devoted to retinal vessels in children. Investigation of blood vessels in infants with ROP was conducted only in the active phase of the disease, and the importance of the progressive changes in caliber and tortuosity of vessels was confirmed for the prognosis of the disease and the choice of tactics of treatment.14−16 A detailed analysis of retinal vessels in cicatricial ROP has not been previously performed. Considering that cicatricial/regressive ROP has a great polymorphism of clinical manifestations, it is necessary to evaluate the role of the vascular component in the pathogenesis of late complications of this disease.
Changes of the standard electroretinogram (ERG)-the maximum, scotopic, photopic, flicker (FERG), at different stages of ROP are well described by many authors.6-9 Glial index (b-wave/FERG 12 Hz), described by Zueva MV and Tsapenko IV in 1992-2009, reflects the state of glial cells in various degenerative and proliferative retinal conditions such as AMD, diabetic retinopathy, retinal detachment10-12 but it has not been studied well at ROP. Considering rather large avascular zones or coagulates in the periphery of the retina that could significantly affect the results of the study, using FERG at 12 Hz for the calculation of the glial index in patients with cicatricial ROP is not appropriate in our opinion. 30 Hz FERG, in contrast, is more stable and easy to use, because requires no changes to the standard examination protocol. AB-index (also called b/a ratio) was suggested by I. Perlman as an additional method in assessment of the retina.13 It is based on physiological considerations regarding the origin of the ERG components. In children with ROP this method can be used to assess retinal blood flow by evaluating the difference in function of outer and inner retina. At the present stage, despite the active introduction of morphometric techniques in ophthalmic practice only individual papers are devoted to retinal vessels in children. Investigation of blood vessels in infants with ROP was conducted only in the active phase of the disease, and the importance of the progressive changes in caliber and tortuosity of vessels was confirmed for the prognosis of the disease and the choice of tactics of treatment.14-16 A detailed analysis of retinal vessels in cicatricial ROP has not been previously performed. Considering that cicatricial/regressive ROP has a great polymorphism of clinical manifestations, it is necessary to evaluate the role of the vascular component in the pathogenesis of late complications of this disease.
ROP findings
We examined 58 children (82 eyes) aged from 6 months to 16 years old at the moment of the first examination and followed them for 5 years (Figure 1). All surveyed children were born of premature birth on gestational age 26-36 weeks (average 29 weeks) and weighing 830-2920 g. (average 1377 g). Prophylactic treatment in active phase was performed on 46 eyes (56.1%). Visual acuity was from 20/20 to 20/400 (mean 20/70). All patients had no general complaints at the time of investigation. Ophthalmoscopic changes in cicatricial ROP included residual avascular zones (82 eyes, 100%), the shift of the vascular bundle (81 eyes, 98.8%, (Figure 2), extraretinal tissue (52 eyes, 63.4%), retinal folds (35 eyes, 42.7%, (Figure 3), degenerative and atrophic focuses (36 eyes, 43.9%), tractional retinoschisis (31 eyes, 37.8%, (Figure 4).
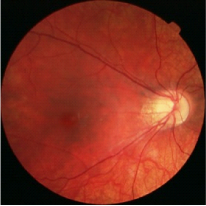
Figure 3 Fundus image of cicatricial ROP with shift of the vascular bundle, macular ectopia and epiretinal fibrosis in central zone.
OCT findings
Normally, arteries and veins are localized in the retina ganglion cell layer (GCL).17 Location of blood vessels in our patients was pertaining to the inner surface of the retina. In 12 of 82 eyes (15%) retinal vessels were in GCL (Figure 5). In 29 eyes (35%)-they were located in the nerve fiber layer (NFL), and in 41 eyes (50%)-extraretinal (on the surface of the retina, (Figure 6). Thus, only 15% of cases demonstrated a physiological, GCL location of vessels. We have studied the localization of the retinal vessels in various clinical situations (Table 1). There was a relation between the degree of displacement of retinal vessels and presence of extraretinal tissue, as well as a tendency to thinning of the retina in cases with extraretinal localization of the vessels (p <0.01). Interconnection between gestational age and birth weight with the position of vessels in the retina was not found (p> 0.05). We analyzed the dependency of the frequency of late complications of cicatricial ROP, such as atrophic changes and traction retinoschisis, with the depth of retinal vessels bedding (Figure 7). The Figure 7 shows that the cicatricial ROP proceeded without complications in a quarter of cases at the localization of vessels in GCL (4 eyes) and NFL (7 eyes), and only in 5% of the cases with extraretinal localization (2 eyes). Atrophic changes were more frequent in cases with localization of vessels in GCL (67%, 8 eyes), and traction retinal detachment and retinoschisis –in cases with extraretinal localization (63%, 26 eyes). Atrophy and traction retinoschisis met with the same frequency in cases with localization of retinal vessels in NFL-38% (11 eyes).
Localization of retinal vessels |
Gestationalage (weeks) |
Birth weight (grams) |
Thickness of neuroepithelium in a zone I (microns) |
Presence of extraretinal tissue |
GLC |
30±0,8 |
1512±126 |
285±13 |
50% |
NFL |
29,9±0,5 |
1431±99 |
280±7 |
76% |
Extraretinal |
29,3±0,3 |
1294±52 |
239±8 |
90% |
Table 1 Localization of retinal vessels in various clinical situations
ERG findings
We conducted a study of changes of maximal and flicker ERG in different variants of the localization of retinal vessels and the results were similar to the previous studies of ERG in children with history of ROP,6,9,13,14 however, there were no statistically significant differences in the standard amplitude-time parameters of the ERG (Table 2). However the study of AB-and glial index revealed interesting patterns (Figure 8). The glial index was within normal limits when the localization of the retinal vessels was within the GCL and/or the NFL and significantly increased when the localization was extra-retinal. The AB-index was reduced in the presence of retinal vessels in the GCL and when measured with other variants of retinal vessel localization remained within normal limits. To investigate the connection of these changes with the development of late complications in the cicatricial ROP, we examined the AB-and glial indexes at different vitreochorioretinal changes (Figures 9 & 10). When cicatricial ROP was uncomplicated, AB-index was reduced and glial index was within normal limits. In cases with atrophic changes both indexes were reduced. At the development of traction retinal detachments and retinoschisis AB-index was within normal limits and glial index was significantly increased.
GCL |
NFL |
Extra |
Norma |
|
a-wave amplitude |
9,3±2,2 |
13,5±3,1 |
8,3±0,9 |
17 |
a-wave latency |
27,9±2,3 |
29,5±0,9 |
26,4±1,6 |
26 |
b-wave amplitude |
30,4±8,5 |
51,2±5,4 |
43,8±6,1 |
165 |
b-wave latency |
56,4±3,2 |
59,0±1,4 |
54,6±2,9 |
61 |
flicker ERG 30 Hz |
6,5±2,3 |
8,0±0,8 |
4,5±0,5 |
16 |
Table 2 Standard amplitude-time parameters of the ERG in different variants of the localization of retinal vessels
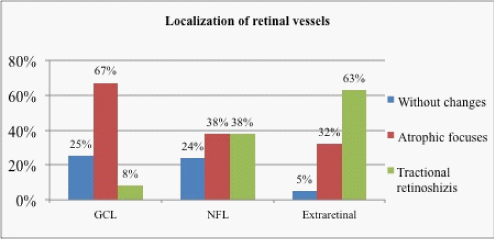
Figure 8 The incidence of vitreochorioretinal changes in different variants of the localization of retinal vessels.
The outer layers of the retina are supplied by choroidal blood vessels while the inner layers of the retina are supplied by retinal blood vessels.18 Displacement of the retinal vessels may cause degenerative processes in the retina. Factors contributing to the displacement of retinal vessels are unknown. In our opinion, a possible theoretical basis for this displacement exists. For example, immaturity of retinal structures due to the aborted embryogenesis and retinal thinning, could lead to the eventual “expulsion” of blood vessels due to a mismatch between the biomechanics of the tissue. Also pathological neovascularization (with eventual vitreoretinal proliferation) may cause tightening, pre-expulsion, and if unchecked, expulsion of the retinal vessels from the retinal surface. In our opinion, the theoretical basis of this displacement can be immaturity of retinal structures in connection with the aborted embryogenesis, thinning of the retina, leading to the “expulsion” of blood vessels, due to mismatch of biomechanical characteristics of the tissue, pathological neovascularization and vitreoretinal proliferation causing “tightening” of the retinal vessels to the retinal surface. Analysis of the correlation of the degree of the displacement of vessels, gestational age and body weight showed that the degree this shift does not depend on the degree of prematurity of a child (p>0.05). The study of correlation between the position of vessels and thickness of the central retina showed that extraretinal localization was characterized by significant thinning of the retina (p<0.01). In other variants of the location of vessels significant deviation from the physiological retinal thickness was absent. Investigation of the depth of the vascular bedding and the presence of extraretinal tissue showed marked direct correlation between these parameters. Analysis of late complications in children with cicatricial ROP revealed a relationship between frequency and nature of vitreochorioretinal changes and depth of retinal vascular bedding. Atrophic changes are common to all variants of the localization, but the incidence of tractional retinal detachments and retinoschisis increases with increasing degree of the displacement vessels.
The method of calculation of glial index, described by Zueva MV and Tsapenko IV, was based on the fact that Muller cells contribute to the b-wave of a single flash ERG,19 but these cells cannot produce rhythm faster than 2 Hz. Therefore, they suggested a glial index as the ratio of the single flash ERG b-wave amplitude to the 12 Hz FERG amplitude. Considering changes in the periphery of the retina (avascular zones, coagulates etc.) that could significantly affect the results of the study, using FERG at 12 Hz for the calculation of the glial index in patients with cicatricial ROP is not appropriate in our opinion, because 12 Hz ERGs are depressed by interactions between ON and OFF retinal pathways,20 which could be changed in eyes with ROP.21 30 Hz FERG, in contrast, is more stable and easy to use, because requires no changes to the standard examination protocol. This interconnection between frequency and nature of vitreochorioretinal changes and depth of retinal vascular bedding is supported by electrophysiological studies-changes of AB-and glial indexes. These parameters were decreased at the presence of atrophic changes and considerably increased (especially glial index) at the development of traction retinoschisis. The study of these parameters in different variants of the localization of retinal vessels showed a tendency to growth of both indexes with the increasing degree of displacement of the vascular bedding, which corresponds to the nature and frequency of late complications in cicatricial ROP.
The analysis of retinal vessels in children with ROP revealed a shift of the vascular bedding to the inner retinal layers with a tendency to extraretinal growth and interdependence of the degree of this displacement to the presence of extraretinal tissue and thickness of the retina. The study showed interconnection between the localization of retinal vessels, changes of AB-and glial ERG indexes and the development of late complications in cicatricial ROP. Thus, these parameters and test results may represent important indicators of the severity of the cicatricial ROP disease process. If determined to be reproducible, these results may indicate a potentially reliable signal of a pre-clinical negative trend in cicatricial ROP.
This study was based on using modern methods of OCT and ERG (including calculation of different indexes). The study allowed defining displacement of retinal vessels bedding and electrophysiological criterions of later complications of regressive ROP (atrophic changes, tractional retinal detachment etc.).
None.
No conflict of interest. No financial disclosure. Presentations at ISCEV 2012 symposium and World ROP Congress III.

©2014 Katargina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.