Advances in
eISSN: 2377-4290


Review Article Volume 1 Issue 4
1Department of Cardiovascular, Respiratory, Nephrologic, Anesthesiologic and Geriatric Sciences, University Sapienza of Rome, Italy
2Department of Biology and Biotechnology, Charles Darwin University, Sapienza of Rome, Italy
3Department of Sense Organs, University Sapienza of Rome, Italy
4Sooft Italia, SpA, Italy
Correspondence: Dario Rusciano, Sooft Italia S.p.A., Via Salvatore Quasimodo 136, 00144, Rome, Italy
Received: October 15, 2014 | Published: December 6, 2014
Citation: Pescosolido N, Pascarella A, Buomprisco G, et al. Critical review on the relationship between glaucoma and alzheimer’s disease. Adv Ophthalmol Vis Syst. 2014;1(4):112-123. DOI: 10.15406/aovs.2014.01.00024
A third of people affected by primary open angle glaucoma (POAG) have normal or even low intraocular pressure (IOP), such as in the case of normotensive glaucoma and low pressure glaucoma. Therefore, high IOP, while remaining the most important risk factor for POAG development, is not the only one. POAG has been recognized to belong to the class of neurodegenerative diseases, such as Alzheimer’s (AD) and Parkinson’s, and several recent studies have suggested possible links between Alzheimer’s and glaucoma both from an epidemiological and a pathogenetic point of view. In fact, several pathogenetic factors are common between the two diseases: the diffuse axonal degeneration of ganglion cells, the presence of specific markers (such as amyloid-β) in the aqueous humor, the activation of caspases and the abnormal processing of amyloid precursor protein in retinal ganglion cells, the reduction of the intracranial pressure of the cerebrospinal fluid, the mutations of the gene that encodes for optineurine. Conflicting data have been reported concerning apolipoprotein E. Finally, numerous publications affirm the utility of molecules traditionally used in the treatment of neurodegenerative diseases (e.g. forskolin, L-carnosine, homotaurine, folic acid, acetylcholinesterase inhibitors, antioxidant vitamins, glatiramer acetate, etc.) also in the treatment of glaucoma. In the present research we analyze the common pathogenetic pathways of Alzheimer’s disease and Glaucoma, we discuss the two diseases with respect to their differences and similarities and we speculate about common therapies, based on the similar pathogenetic findings.
Keywords: amyloid-β, alzheimer’s disease, glaucoma, neurodegeneration, neuroprotection
POAG, primary open angle glaucoma; IOP, intra-ocular pressure; APP, amyloid precursor protein, RGCs, retinal ganglion cells; CSF, cerebro-spinal fluid; APOE, apolipoprotein E; AD, alzheimer’s disease; EGS, european glaucoma society; RNFL, retinal nerve fiber layer; GON, glaucomatous optic neuropathy; PD, parkinson’s disease; NMDA, N-Methyl D-Aspartate; BDNF, brain-derived neurotrophic factor; EOFAD, early-onset familial AD; MCI, mild cognitive impairment; PET, positron emission tomography; SPECT, single-photon emission computed tomography; MRN, magnetic resonance neurography; CT, computed tomography; TNF-α, tumor necrosis factor α; OCT, optical coherence tomography; DKEFS, delis-kaplan executive function system; GDS, geriatric depression scale; GAI, geriatric anxiety inventory; CVLT-IIED, california verbal learning test- second edition; PERG, pattern electroretinogram; NTG, normo-tensive glaucoma; HHcy, hyper-homocysteinemia; AβPP, amyloid-β protein precursor; FDA, food and drug administration; ON, optic nerve; OAG, open angle glaucoma; CSI, conformational-selective interference; ER, endoplasmic reticulum; EGR-1, early growth response 1; EGCG, epigallocatechin gallate; ERG, electroretinogram; CAMP, cyclic adenosine monophosphate; AH, aqueous humor; PKA, protein kinase A; ROS, reactive oxygen species
Until recently, POAG and AD had always been described as two distinct pathologies, despite similar pathophysiology and demographic distribution. Both diseases are neurodegenerative, chronic and progressive in nature, with irreversible neuronal cell loss. Furthermore, both POAG and AD disease primarily affect the elderly, with a strongly age-dependent incidence. The progressive debilitating course of both diseases has tremendous implications on an aging population.1 For years Glaucoma has been associated with high IOP,2 although scientists had accepted this etiology with caution, since it was known that POAG could develop in subjects with normal or even low IOP. In fact, one out of three POAG patients has normotensive or low tension glaucoma.3 This observation has led the scientific community to look for other possible causes of this slowly debilitating pathogenesis.
The hallmark of neurodegenerative diseases is the death of specific neuron populations and of those other neurons that are inter-connected and depend for their survival on the inputs from the main population (trans-synaptic degeneration). In Parkinson’s disease accumulation of alfa-synuclein4 causes the degeneration of dopaminergic neurons of the substantia nigra, in the midbrain, thus leading to the classic symptoms of Parkinson’s such as tremor, akinesia, bradykinesia, and stiffness.5 In AD there is a loss of neurons in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.6 Both amyloid plaques and neurofibrillary tangles are clearly visible by microscopy in the brains of those afflicted by AD.7 Plaques are dense, mostly insoluble deposits of beta-amyloid peptides and cellular material outside and around neurons. Tangles (neurofibrillary tangles) are aggregates of the microtubule-associated protein tau which has become hyper-phosphorylated and accumulates inside the cells themselves, disrupting the intracellular transport system. Beta amyloid plaques and/or neurofibrillary tangles trigger apoptosis of neuronal cells, thus causing the disease.8
POAG results from the death, mainly by apoptosis, of RGCs, which in turn causes, by trans-synaptic degeneration, the shrinking of the lateral geniculate nuclei, and finally of the occipital cerebral cortex.9 It is not yet clear what initiates the death cycle of RGC; it could be an effect of high IOP, sometimes due to a malfunction of the trabecular meshwork and the lamina cribrosa, and/or also a true neurodegenerative mechanism, triggered by the toxic accumulation of some metabolites. In favor of this hypothesis, some studies have reported that amyloid-beta, a substance found in cerebral tissues of AD patients, is also implicated in RGC death.10 Conversely, other studies have shown a relatively higher occurrence of POAG in AD patients.11−18
Primary open angle glaucoma: more than just high pressure
Glaucoma, according to the updated definition of the European Glaucoma Society (EGS), is a “chronic optic neuropathy with morphologic alterations of the optic nerve head and RNFL which causes alteration of visual perimetry without any other eye pathology”. IOP higher than normal (>21 mmHg) is still considered the main risk factor for POAG development. Several types of Glaucoma are known; these can be divided in primary and secondary. Primary glaucomas have an intrinsic etiology, depending on physiological or anatomical defects of the optic system; secondary glaucomas depend on extrinsic causes, such as drugs (iatrogenic glaucoma, e.g. after intravitreal steroid injection) or traumas. Primary glaucoma can occur with a closed iridocorneal angle (acute closed-angle glaucoma), or with an open angle. The most prevalent form of glaucoma is the primary open angle glaucoma, in which the iridocorneal angle, the main way for the outflow of aqueous humor, is freely accessible, but the draining tissue-the trabecular meshwork-may become less permeable.
There are two main types of POAG: one that occurs with an IOP higher than normal (>21 mmHg) and represents 66% of the total POAG and the other occurring with an IOP <21 mmHg, which can be normal (normotensive glaucoma) or even lower, than normal (low tension glaucoma). However, also in these latter cases, the main therapy still relies upon a further IOP decrease. Glaucoma is a neurodegenerative disorder caused by RGC apoptosis, due to a variety of complicated mechanisms. According to various theories, factors including elevated IOP and vascular dysregulation19 primarily contribute to the initial insult during glaucomatous atrophy in the form of obstruction of axoplasmic flow within RGC axons, alteration in optic nerve microcirculation at the level of lamina and changes in the laminar glial and connective tissues. Deprivation of neurotrophic factors, such as the BDNF, and release of a large number of neurotoxic agents within the retina, including glutamate, nitric oxide and free radicals, are the consequences of the above-mentioned events. Currently, IOP reduction is the only proven clinical therapy available for the treatment of glaucoma. However, clinicians know that signs of progression can be seen in many patients despite well controlled IOP. In addition, glaucomatous optic neuropathy has been observed in patients with ‘normal’ IOP. Therefore, the pathophysiology of glaucoma and particularly normal tension glaucoma probably goes beyond IOP alone. Studies on low pressure glaucoma have demonstrated that in most patients high intraocular pressure is unrelated to poor visual fields.
Over the past decade, most studies have focused on assessing neuroprotective treatments along with IOP lowering medications in order to prevent ganglion cell death or even reverse the process of cell death. Neuroprotection aims at blocking primary destructive events affecting RGCs or optic nerve fibers, enhancing their survival mechanisms and finally repairing damage occurring during the progressive, secondary stage of the injury.20 Direct neuroprotection protects cells against intrinsic mechanisms of cell death and apoptosis, such as an excess of free radicals, glutamate excito-toxicity, or imbalance of cell survival/death factors. Indirect neuroprotection protects the cells from local damaging environmental events such as elevated IOP and low vascular perfusion.21 Neuroinflammation has also been associated with POAG22 because it appears to be a major contributor to the development of other chronic neurodegenerative diseases beside POAG, such as AD and PD.22 Therefore, research in these recent years has been focused on addressing the similarities between the different slow progressing neurodegenerative diseases, to see whether it could be possible to find a common mechanism of neuron cell death that could be counteracted by specific drugs or natural molecules, according to a neuroprotection strategy.
A promising medical approach for glaucoma neuroprotection resided in glutamate antagonists. These molecules are able to protect cell membranes against the hyperactivity of NMDA receptors. In particular memantine, being well-tolerated, has been widely used in AD, and its neuroprotective properties have been demonstrated in an animal model of RGC death.23 However, the largest randomized phase III clinical trial concerning the efficacy of memantine for POAG showed no significant benefits.24−26 In 2009 Nahagara et al.27 demonstrated that neurotrophins (and in particular BDNF) have neuroprotective effects in several (transgenic mice, adult rats and adult primates) animal models of AD. Moreover the use of stem cells bioengineered to over express BDNF has shown to protect RGCs in a rat eye model of hypertension;28 on the other hand, Chader29 affirms that neurotrophins can only delay cell death, without providing a real cure. Recently researchers have investigated anti-inflammatory agent properties in counteracting neuronal damage. Szekely and Zandi in 201030 reviewed several epidemiological studies in order to clarify if non-steroidal anti-inflammatory drugs may be neuro-protective, and found the evidence controversial. A more promising approach is provided by the use of antioxidants, because various degenerative disorders are associated with oxidative stress. In particular, for glaucoma it seems that oxidative stress may induce RGC death through damage of the trabecular meshwork, the optic nerve head and the retina. Among antioxidant molecules, vitamin C, vitamin E and Ginkgo biloba have been extensively studied.31−33
Finally, in 2003 Bachurin et al.34 were among the first to propose a role for mitochondria in neuroprotection. In fact, they observed that beta-amyloid protein is able to induce the opening of mitochondrial pores that, in turn, play an important role in neurotoxicity. Three years later Osborne et al.35 proposed the hypothesis that RGCs in POAG exist at a low energy state because of low blood perfusion, so that their ability to get rid of the excess of ROS produced by axon mitochondria when they are directly hit by short wavelength radiation is impaired, thus resulting in oxidative stress and finally apoptotic cell death. Such hypothesis has been confirmed in further reports,36−38 leading to the suggestion that supplementation with creatine, alpha-lipoic acid, nicotinamide and EGCG could be protective for mitochondria,39,40 and useful to treat neurodegenerative pathologies. Therefore, the improvement of neuronal mitochondrial respiration could be a promising approach for neuroprotection.
Alzheimer’s disease and its pathogenesis
AD is the most common type of dementia; it has a chronic progression and involves about 5% of people over 65 years of age.34 Currently it affects 26million people around the world, and it is estimated that in 2050 there will be over 100million of affected individuals.41 The risk of contracting the disease, in fact, increases with age: it has been calculated that nowadays about 20% of the population aged over 85 is affected by AD. However, there are rare cases of people who may have an early onset of the disease before the fourth decade of life (EOFAD: early-onset familial AD).
The course of the disease and its clinical manifestations are highly variable.
Three main stages can be identified:
The disease can also be preceded by a pre-dementia stage, called MCI (Mild Cognitive Impairment), a kind of cognitive decline, frequent in the elderly population, but not necessarily indicative of an incipient dementia. Several conditions are now proven risk factors for AD; among these are diabetes, obesity and hypercholesterolemia.41 The diagnosis of AD is a probabilistic diagnosis that is based, currently, on a series of tests. First level tests can be questionnaires designed to assess the intellective, emotional and functional status of the subject, such as the Mini Mental State Examination (Figure 1).42 Second level tests include PET, SPECT, MRN and CT43 (Figure 2).
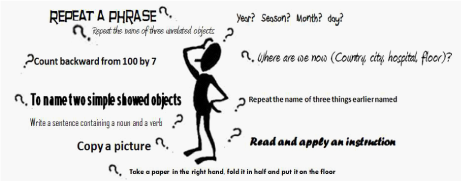
Figure 1 Mini Mental State Evaluation (MMSE) test is used to assess mental and cognitive status. It is an 11-question test that scores for orientation, retention, attention, recall and language. The maximum possible score is 30, representing high function. A score of 23 or lower is indicative of cognitive impairment.
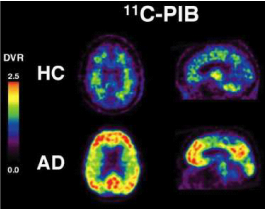
Figure 2 Transaxial and sagittal distribution volume ratio (DVR) of the Aβ-ligand11 C-PIB. PET images of a representative healthy control (HC) subject (age: 84, MMSE: 30) and an Alzheimer’s disease patient (age: 83, MMSE: 22). Image credit to Dr Victor Villemagne and Associate Professor Chris Rowe, PET Centre, Austin Health, Heidelberg, Australia.
The pathogenesis of the disease44 is characterized by progressive neuronal death mainly due to the accumulation in the extracellular space of the β-amyloid protein (Figure 3), which is produced by the predominant action in these patients of the enzyme β-secretase on the APP. In healthy subjects the APP is cleaved instead by α-secretase, and does not generate the insoluble precursor β-amyloid (Figure 4). β-amyloid tends to form insoluble aggregates (amyloid or senile plaques) that are deposited in extracellular spaces (Figure 3) and trigger an inflammatory process (production of cytokines, interleukins and TNF-α, activation of immune cells etc.) finally leading to neuronal death. Moreover, beside the amyloid plaques, the abnormally phosphorylated tau protein also forms insoluble structures, the neurofibrillary tangles (Figure 5) that are pathognomonic for AD. A third characteristic pathogenetic element in AD is amyloid angiopathy45 a form of angiopathy in which amyloid deposits form in the CNS blood vessel walls46 thus increasing the risk of cerebral micro-hemorrhages.
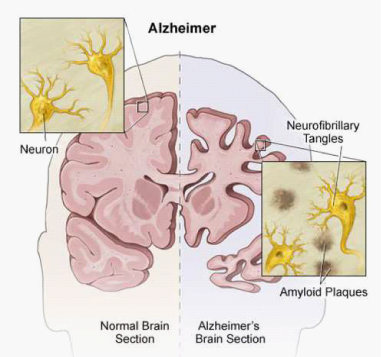
Figure 3 An illustration comparing a normal brain and a brain affected by Alzheimer’s disease. Amyloid plaques (abnormal clusters of protein fragments) build up between nerve cells, whereas neurofibrillary tangles made up of Tau protein grow inside neurons. Image credit to: student osteopathic medicine.tumblr.com
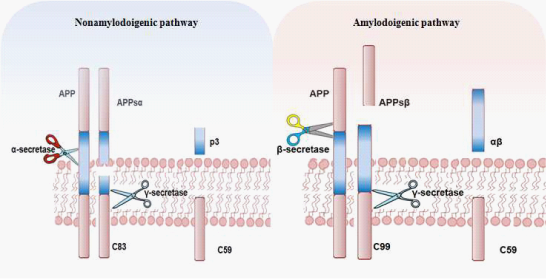
Figure 4 The amyloid precursor protein (APP) is a transmembrane protein that can undergo a series of proteolytic cleavages by secretase enzymes. When it is cleaved by α-secretase in the middle of the β-amyloid domain, it is not amyloidogenic. However, when APP is cleaved by β-and γ-secretase enzymes, insoluble neurotoxic Aβ peptides are released, which can accumulate in oligomer aggregates..
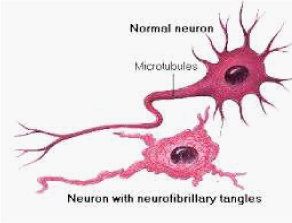
Figure 5 Difference between normal and Alzheimer’s neurons. Top: normal neuron without Alzheimer’s. Bottom: Deposition of neurofibrillary tangles in brain nerve cells with Alzheimer’s disease. Image credit to Nation Institute of aging. Illustrator Lydia Kibiuk
AD is characterized by the loss of neurons and synapses in the cerebral cortex and certain subcortical regions, leading to degenerative changes in a variety of neurotransmitter systems. These include alterations in the function of the monoaminergic neural systems that release glutamate, norepinephrine, and serotonin as well as other neuropeptide-containing systems. This neuron loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.6 The destruction of neurons then leads to a progressive, diffuse cerebral atrophy (Figure 6), the total volume of the brain is reduced and the parenchyma is replaced by CSF that expands the ventricle (Figure 7) and may lead to normotensive hydrocephalus.47
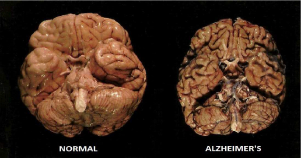
Figure 6 Comparison of a normal human brain and a brain from a patient who died with AD. Image credit to sumitachakraborty.com
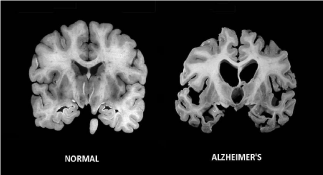
Figure 7 Massive cell loss changes the brain in advanced Alzheimer’s disease. Image credit to going gentle into that goodnight.com
Alzheimer’ disease and eye pathologies
Often, eye involvement is an early occurrence in AD.48−50 There are reports describing reduction of perimetric indexes,51,52 contrast sensitivity,53,54 perception of movement55,56 and colors.57 Previously, all these defects were considered to be due to pathological changes in the cortex. However, the development of modern ocular imaging techniques has shown that pathological changes within the retina could also be found in these patients.58 Moreover, since AD is known to be characterized by a dysfunction of the cholinergic system, which also regulates the pupillary diameter, several studies have explored and demonstrated a hypersensitive response of AD patients to both mydriatic (e.g. tropicamide) and miotic (e.g. pilocarpine) drugs.59−67 Some have even proposed that this altered response could be pathognomonic of AD68−71 while others have refuted this hypothesis as non-specific.72 The crystalline lens also could be involved in AD, and high levels of β-amyloid were described in lens epithelia of subjects with a particular and rare type of cataract, the anterior subcapsular cataract.73,74 Other authors have investigated possible changes in retinal anatomy in patients with AD, reporting an abnormal reduction of retinal thickness (by OCT)75 associated with defective blood flow.76 This retinal damage appears to be associated with increased amounts of b-amyloid and tau proteins in the vitreous body.77 Finally, a reduction in the number of optic nerve fibers, probably due to the toxicity on RGCs of β-amyloid, has been detected in some studies.78−80
Glaucoma and Alzheimer’s disease, from epidemiological relationship to common pathogenetic mechanisms
Studies suggesting a possible link between Alzheimer’s disease and glaucoma have sought this relationship studying either epidemiological data, or pathogenetic events that occur in both conditions (Figure 8). Chandra and colleagues81 first described a high frequency of glaucoma occurrence in patients with presenile and senile dementia, after analyzing thousands of death certificates in the United States from 1978 to 1986. In 2002, Bayer et al.82,83 described a series of 49 patients with AD, 12 of which (24.5%) presented with diagnostic criteria for glaucoma, having visual field defects with a cup/disk ratio>0.8. This finding was subsequently confirmed and extended in a study with a larger case series: 112 subjects with AD, 29 of them were consistent with the diagnosis of glaucoma; this represented a prevalence of 25.9% in this group, compared to a prevalence of 2.6-4.7 % in the same age class of the general population. In a control group of 116 subjects living in the same environment, the incidence of glaucoma was 5.2%, not dissimilar from that of the general population. Yochim and colleagues84 evaluated 41 patients with glaucoma (age 70±9.2 years, 70% females) with a series of questionnaires (DKEFS, GDS, GAI , CVLT-II ED) to assess their cognitive status; 44% of the subjects showed an obvious cognitive impairment, and 12.2% had MCI. PERG is an electrophysiological test that analyzes the electrical response of RGCs, and has been proposed as a test for the diagnosis and follow-up of early POAG alterations. In a retrospective analysis, Nesher and Trick85 compared the PERG of 42 patients with glaucoma, 13 patients with AD, 58 patients with diabetes mellitus and 92healthy control subjects and observed a similarity of alterations in the recordings of patients with AD and glaucoma.
Figure 8 Several studies suggest possible common etiopathogenic factors existing between POAG and Alzheimer’s disease.
However, conflicting data have been reported among the different studies carried out to compare glaucoma frequency in normal and AD subjects. Kessing et al. in 200786 analyzed the hospital records in Denmark from 1977 to 2001, and measured the average incidence of AD in 11,721 patients with POAG and in other patients with other diseases typical of old age (cataracts, arthritis, etc.), and did not find statistically significant differences. Similarly, Bach-Holm et al.17 did not find comorbidity between the two pathologies, and the same was true for a study published in 2012 by Ou et al.18 Taken together, all these observations suggest nonetheless the idea of similar pathogenetic mechanisms between AD and glaucoma, two diseases in which neuronal cell death plays an important role in pathogenesis and prognosis. In fact, Hinton and colleagues87 found widespread axonal degeneration of RGCs in the optic nerves of 8/10 patients with AD. Sadun and Bassi further reported that in 10 patients with AD this loss was predominant among the most numerous class of retinal ganglion cells, the M cells, reporting an observed loss between 30% and 60%.88
The study of Janciauskiene et al.89 is interesting, in this context. It demonstrated the presence of specific markers of AD, such as amyloid-β, in the aqueous humor of patients suffering from glaucoma. Furthermore, it seems that the activation of caspases and the transformation of the abnormal APP, important events that occur in AD, also occur in the RGCs of rats with experimental glaucoma. In 2007 Guo et al.90 demonstrated that amyloid-β is upregulated in the hippocampus of AD patients and RGCs of a glaucoma rodent model. Moreover, intravitreal injection of amyloid-β induces apoptosis in a dose dependent manner. Reducing the level of amyloid-β decreases glaucomatous RGC apoptosis in rats.90 Other conflicting data concerned the APOE, which is heavily involved in the neuronal degeneration characteristic of AD, but is not yet convincingly associated with glaucoma. The inheritance of a particular polymorphism of the APOE gene, carried by allele 34, according to some authors,91,92 has been shown to be associated not only with a high risk for AD, but also for glaucoma; another paper by Kountouras93 supported this association, whereas other published studies by Higuchi63 and Tamura14 excluded it.
Some authors94−96 suggested an association between decreased intracranial pressure due to CSF, which is a common finding in AD, and NTG; however, Tsukahara97 found normal intracranial pressure in a cohort of patients with NTG, and rejected this hypothesis. The Tau protein has also been suggested as a possible common etiopathogenic factor, since it is present both in the CSF of AD patients98 and in the horizontal cells in the retina of glaucoma patients.99 However, the most promising studies indicate the amyloid-β protein as the common pathogenetic factor for both diseases10,11,77,90,100 In fact, this aberrant protein was found in the vitreous humor of patients with glaucoma and seems to be involved in RGC death. Finally, other authors suggested a role played by Helicobacter pylori infection,101,102 or by mutations in the gene that encodes for optineurin.16 The first hypothesis does not seem to have many followers, whereas research on optineurin recently provided a clue that this protein may be indeed a new common underlying risk factor involved in both NTG and AD.103 Optineurin is a hydrophilic, cytosolic protein10 that is ubiquitously expressed in brain and in ocular tissues including the retina, trabecular meshwork and non-pigmented ciliary epithelium. In the retina, RGCs are immunolabeled with high intensity.104,105 Interestingly, optineurin was found in neurofibrillary tangles and dystrophic neurites in AD, thus suggesting a new hypothesis for neurodegeneration in AD involving chronic optineurin neurotoxicity and mimicking NTG at the molecular level.106
Another common risk factor seems to be hyper-homocysteinemia (HHcy).107−109 Homocysteine is a non-protein amino acid and a metabolic precursor of both methionine and cysteine.110 Recent epidemiological studies have found that mild to moderate HHcy, is also a risk factor for sporadic AD.111−113 Zhuo and Praticò in 2010114 measured homocysteine levels and amyloidogenesis in Tg2576 mice (a well-established mouse model of AD-like amyloidosis115) fed with a diet containing excessive methionine and deficient in vitamins of the B group, in order to increase homocysteine levels in vivo.116−117 After 7 months on this diet, they found a severe HHcy in Tg2576 mice, but the diet failed to cause any significant alterations in Aβ levels, deposition, or AβPP metabolism. However, a recent clinical study on a cohort of patients with POAG or pseudoexfoliation glaucoma118 failed to find a correlation between POAG and plasma homocysteine levels. Therefore, further studies are needed to clarify the role of HHcy in neuronal pathologies.116,119
Therapeutic strategies for alzheimer’s disease
Acetylcholinesterase inhibitors: The key existing treatments for AD are symptomatic rather than disease modifying in nature. Acetylcholinesterase inhibitors such as donepezil, rivastigmine and galantamine are well-established symptomatic treatments for AD. They are recommended as first-line therapy by the Quality Standards Subcommittee of the American Academy of Neurology for the symptomatic treatment of AD.120 The rationale for the development of these drugs derives from the cholinergic hypothesis, suggesting that cognitive impairment in AD is the result of cholinergic synaptic dysfunction in the hippocampus and neocortex, caused by early cholinergic neuron degradation.120 Acetylcholinesterase inhibitors work by inhibiting degradation of acetylcholine released into synaptic clefts, thereby improving cholinergic neurotransmission. A recent Cochrane review concluded that donepezil, rivastigmine and galantamine are efficacious in mild-to-moderate AD.121
The therapeutic role of acetylcholinesterase inhibitors in AD poses the question of whether a therapeutic benefit may also exist in glaucoma. A significant proportion of NTG patients have been found to have an AD-like cerebral blood flow pattern.122 In this group of patients, a subsequent pilot study found that oral donepezil significantly improved ON head and cerebral blood flow as well as visual field defects.123 Furthermore, some animal studies demonstrated that donepezil is able to reduce IOP when applied topically, and to significantly reduce IOP in normotensive AD patients after 4 weeks of oral intake.124 Recently, it was also demonstrated that galantamine protects RGC structure and function in a rat model of glaucoma.125 Protection was mediated through the activation of muscarinic acetylcholine receptors, and was independent of IOP. All these studies suggest that acetylcholinesterase inhibitors may have a neuroprotective role in glaucoma. However, further investigation is needed as the evidence to date remains sparse.
Glutamate modulation: Glutamate is a principal excitatory neurotransmitter in the brain. It normally binds to its NMDA receptor on postsynaptic cells to mediate normal excitatory neuronal activity: this induces long-term potentiation, which is related to memory and learning. In AD, excessive glutamate levels and sustained low activation of NMDA receptors lead to neuronal excitotoxicity, which is hypothesized to play a key role in AD.126 This is due to an excessive calcium influx through the receptor-associated ion channel and subsequent free radical formation, which in turn leads to neurodegeneration.127 A number of factors, such as Aβ oligomers128,129 and ApoE-ε4130 have been demonstrated to play a role in this mechanism. Various agents have been developed to modulate glutamatergic neuro-excitotoxicity. Among these, the US FDA licensed the use of memantine for the symptomatic treatment of AD.131 It is a noncompetitive, low-affinity NMDA receptor antagonist that preferentially blocks excessive NMDA receptor activation, without disrupting the physiological NMDA receptor activity required for cognitive function.132 There is also preclinical evidence in AD suggesting that memantine may have a neuroprotective role in AD, as well as being a symptomatic treatment.133 It has been shown by randomized controlled trials that memantine can improve cognition, behavior and activities of daily living in people with moderate-to-severe AD,134 even if there is no evidence of a therapeutic benefit in mild AD.135 It should be noted that combined therapy with memantine and donepezil in patients with moderate-to-severe AD resulted in better cognition, behavior, activities of daily living and global outcome as compared with donepezil alone.136 Glutamate-mediated neurotoxicity is thought to also play a role in glaucoma. In fact, the association between RGC death and glutamate toxicity has been shown in several glaucoma animal models.137 Memantine has been demonstrated to be protective against NMDA-induced neuronal loss in vitro.138 Application of memantine in vivo significantly increased RGC survival in a DBA/2J transgenic mouse model of glaucoma,139 in an ischemic rabbit model,140 and in ischemic and hypertensive rat models.141 Memantine has further been shown to protect against structural and functional RGC loss in a monkey model of glaucoma.27 In addition, monkeys affected by glaucoma and treated with memantine demonstrated significantly less neuronal shrinkage in the lateral geniculate nucleus, which is the principal target of RGCs.142 It is noteworthy that memantine is the only neuroprotective drug that has completed a Phase III clinical trial in OAG. Unfortunately, the drug was found to be ineffective with regard to its primary end point of improving the visual field.26 Another noncompetitive antagonist of the NMDA receptor, MK-801, has similar functions to memantine but is not used in AD as it can cause schizophrenic symptoms. MK-801 has been found to protect RGCs in vitro as well as in vivo in various animal models of glaucoma.143
Alternative approaches: The obvious limits of the above treatments led scientists to develop alternative therapeutic approaches. Preclinical experiments in transgenic mice suggested that passive immunization with antibodies against the beta amyloid protein might counteract the progression of AD in humans.144−146 Currently, several conformation- and oligomeric-specific antibodies targeting the Alzheimer’s amyloid beta oligomers (AbOs) have been developed. However, they appear largely not exploitable for subcellular targeting and intracellular functional studies in living cells. In 2014 Meli et al. generated, by an in vivo intracellular selection in yeast cells, a set of conformation-sensitive antibody fragments for different subcellular compartments,147 developing a CSI method. This approach allowed determining the cellular mechanisms of AbO generation, demonstrating that intracellular amyloid bodies can oligomerize into pathological forms through critical conformations formed inside the endoplasmic reticulum (ER). The anti-AbO intrabody selectively intercepts critical AbO conformers and controls their ‘toxic’ assembly in the ER, without interfering with the complex processes of maturation and processing of APP.147
As regards the relationship between AD and POAG, Cheung and co-workers were among the first to suggest that, since the neurodegeneration that affects patients with glaucoma refers to similar pathogenic mechanisms as AD and PD (glutamate excitotoxicity, mitochondrial dysfunction, protein misfolding, oxidative stress, inflammation and deprivation of neurotrophins), then, also from a therapeutic point of view, such neurodegeneration can be dealt with by similar therapeutic strategies.148 In support of this concept, many publications support the use of molecules traditionally employed in the treatment of classic neurodegenerative diseases also in the treatment of glaucoma.149 Bakalash et al.150 evaluated the effect of glatiramer acetate in mouse models of glaucoma demonstrating the increased expression of EGR-1, a nuclear transcription factor involved in the phenomenon of neuronal plasticity with neuroprotective effect. Estermann et al. in 2006124 documented a hypotonic effect by donepezil (Aricept), a selective inhibitor of acetylcholinesterase used in the treatment of AD, on 32 normotensive patients. Tatton et al.149 on the other hand, believe that α2-adrenergic receptor agonists (e.g., brimonidine) have a neuroprotective role both in AD and in glaucoma. Lee and co-workers, in 2012, observed a reduction of β-amyloid in laboratory mice who were given vitamin D3.151 In recent years there has been a growing interest in epigallocatechin gallate (EGCG), a component of green tea, in the context for neuroprotection in glaucoma and Alzheimer’s. In 2008 Osborne39 demonstrated that in a rat model of retinal ischemia due to a sudden elevation of IOP, ECGC is able to prevent apoptosis in ganglion cells by protecting mitochondrial function. These results were further confirmed by Zhang et al.152 who recorded the electroretinograms (ERGs) of eyes from ischemia-induced rats. ECGC properties were also evaluated in humans by Falsini et al.153 and the obtained PERG results suggested that EGCG might favorably influence inner retinal function in eyes with early to moderately advanced glaucomatous damage. It seems that ECGC might also have protective effects on AD: in 2012 He et al.154 evaluated the beneficial effects of ECGC by immunohistochemical analysis of APP in the hippocampus of an AD mouse model. Further, Zhang et al.155 reported that the polyphenol (-)-EGCG is able to inhibit Aβ oligomerization.
Homotaurine also deserves a specific mention. Homotaurine is a molecule that has been extensively investigated as a neuroprotector in AD. It is a small aminosulfonate compound that is present in different species of red marine algae and it has been shown, in both in vitro and in vivo models, to be able to provide a relevant neuroprotective effect by its specific anti-amyloid activity and by its γ-aminobutyric acid type A receptor affinity. Homotaurine may prevent the formation of the insoluble beta-amyloid complex, thus preventing its related neurotoxicity.156 The ALPHASE study, recently published by Aisen et al. in 2011, enrolled 1052 patients affected by AD, and showed that homotaurine treatment may prevent further cognitive decline and loss of memory in these patients, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting disease-modifying effects.157 This functional result is correlated to the anatomical finding of a lesser decrease in the hippocampal volume in those patients treated with the oral supplement of homotaurine. Since amyloid plaques have also been shown to occur in RGC as a consequence of elevated IOP levels158−159 beta amyloid appears to be a possible target in glaucoma neuroprotection.160
Another promising molecule is forskolin, a diterpene extracted from the roots of Coleus Forskohlii, a plant growing in India. It lowers IOP through a receptor-independent increase of cyclic adenosine monophosphate (cAMP) that finally results in a decrease of the inflow of AH in the eye chambers.161 Moreover, the increased levels of cAMP also result in PKA activation and Rho kinase inhibition, in turn causing the disassembly of the actin cytoskeleton in the trabecular meshwork. This process enhances the permeability of the trabecular meshwork to the AH outflow, also contributing to a decrease of IOP.162 Accordingly, topically applied forskolin has been shown to lower IOP in both rabbit and monkey model systems.163 Such an effect has also been replicated in humans by oral systemic treatment, and two different publications21 indicated that forskolin given to POAG patients under maximum tolerated medical therapy is able to exert a further hypotonizing effect, significantly decreasing IOP irrespective of the drug regimen present. Besides its effects on IOP, cAMP is also able to enhance neuronal and RGC survival, and induce specific beta-amyloid phagocytic activities in microglia cells. The anti-apoptotic effects of cAMP are seen in many types of neuronal systems, including cerebellar granule neurons,164−169 dopamine neurons,170 septal cholinergic neurons,171 and sympathetic and sensory neurons.172−174 A clinical study on POAG patients treated with a food supplement containing forskolin and rutin (Kronek®) has shown an improvement of the electrophysiological activity of RGC as measured by PERG analysis.175,176
Carnosine is a di-peptide (alanine + histidine), found in large amounts in the eye lens, the brain (especially the primary olfactory ways) (5mM) and in voluntary muscles (20 mM).177 It has been shown to have neuroprotective effects on RGCs against glutamate and free radical toxicity,178,179 and also attenuate through similar mechanisms β-amyloid neurotoxicity.179 In the context of AD, carnosine has been suggested as a therapeutic agent, given its capability to act as a metal chelator, free radical scavenger as well as an inhibitor of Aβ toxicity.178 In 2011 Corona et al. investigated the potential beneficial effects of dietary carnosine supplementation (10 mM in drinking water) in 3xTg-AD mice,180 an AD animal model that develops an amyloid- and tau-dependent pathology, as well as AD-related cognitive deficits.171−181 When Corona et al. analyzed the effects of carnosine on this mouse model system, they found that carnosine was very effective in decreasing intraneuronal Aβ deposition in the hippocampus. Analysis of the effect of carnosine supplementation on cognitive deficits of 3xTg-AD mice showed a positive trend, indicating that it might also have a beneficial role in preventing long-term memory deficits.180 It is interesting to note that the association of these three molecules (homotaurine, forskolin and carnosine), which is present in a commercial product available in Italy (Gangliolife®), is able to protect RGCs in vitro by two different neurotoxic molecules such as rotenone and sodium azide.181,182 Finally, a new drug potentially useful in the treatment of both diseases is ademetionine, an antidepressant (depression is one of the main psychiatric symptoms of AD) that appears to reduce the aggregation of β-amyloid fibrils183,184 and shows neuroprotective effects on RGCs.185,186
According to what has been illustrated here, the emerging message is an encouragement to healthcare professionals involved in the management of AD (Geriatricians, Neurologists, etc.) to also include, as part of multi-disciplinary assessment of the elderly patient, a systematic ophthalmic examination, aimed at evaluating the risk factors for glaucoma disease and the status of visual function in general. We also need to give serious consideration to the possibility of using certain medications in both of these diseases. Numerous publications, in fact, confirm that what protects the nervous system from neurodegeneration (e.g. forskolin, L-carnosine, homotaurine, folic acid, acetylcholinesterase inhibitors, antioxidant vitamins, glatiramer acetate, etc.), also protects RGCs, delaying the visual decline and improving patients’ quality of life.
The authors acknowledge the critical proof reading of the manuscript by Dr Antony Bridgewood.
N. Pescosolido, A. Pascarella and G. Buomprisco report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article. D. Rusciano is a full time employee of Sooft SpA, the Italian pharmaceutical company that produces and commercializes the food supplement Gangliolife.

©2014 Pescosolido, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.