Advances in
eISSN: 2377-4290


Purpose: The aim of this study is to present subjective patients symptoms present after surgical excision of various epibulbar lesions and their reduction after local use of cyclosporine a cationic solution eye drops.
Methods: A group of patients after the surgery of the epibulbar tumors in the January-March 2017 period. After surgery cyclosporine A (CsA) treatment was administered once a day. We evaluate results 12months of treatment.
Results: A group of 8 patients, an average age of 43years. Indications of treatment: recurrent epibulbar carcinoma-2 patients (25%), dysplastic melanocytic nevus-4 patients (50%), malignant melanoma of the conjunctiva-1 patient (12.5%), epibulbar MALT lymphoma-1 patient (12.5%). We evaluate the course of healing for the first 12 months after surgery. The calculation results confirmed that treatment by CsA after surgery of the melanocytic lesions group is considered to be statistically significant (P value equals 0.0351) and the treatment by CsA after surgery of the non-melanocytic lesions group is considered to be not statistically significant (P value equals 0.1027).
Conclusion: The function of tear film in patients with epibulbar tumors is limited. CsA specifically affects only the function of T lymphocytes, diminishing the production of cytotoxic T lymphocytes. Application of CsA is another option for treating dry eye syndrome and post-surgery complications of epibulbar tumors, which, in the post-surgery course, is especially important in the treatment of the inflammatory component of the post-surgery response.
Keywords: epibulbar, tumor, dry eye syndroma, cyclosporine A, conjunctival lesion
Tumors of the conjunctiva and cornea comprise a large and varied spectrum of lesions, congenital and acquired lesions. Ocular surface tumors include a variety of lesions originating from squamous epithelium, melanocytic tumors and lymphocytic resident cells of the conjunctival stroma. One of the first symptoms in these patients can be dry eye syndroma.
Dry eye is a multi-factorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” This is an actualized definition of the dry eye disease according to the TFOS DEWS II Definition and Classification Subcommittee published in 2017.1 Common symptoms of dry eye disease (DED) include dryness, irritation and foreign body sensation, light sensitivity, increased tearing or itching. The pathogenesis of the disease is not clear.2 Reduced lacrimal tear secretion and volume causes tear hyperosmolarity leading to hyperosmolarity of the ocular surface epithelial cells. This stimulates a cascade of inflammatory events which play an important role.3 Mediators such as cytokines, chemokines, and matrix metalloproteinases promote the activation of immature antigen-presenting cells (APCs). This leads to expansion of autoreactive CD4+ helper T cells followed by self-perpetuating cycle of inflammation.2 Prevalence of the DED is between 5 and 35% according to many published studies depending on diagnostic criteria. Increased prevalence is present in women and older population.4 The severe form of the disease is characterized by persistent and recurrent symptoms that are poorly correlating with the objective clinical findings.5
Tumors of the ocular surface have a wide clinical spectrum and include several forms of epithelial, stromal, caruncular and secondary tumors. Complete excision is the treatment of choice in majority of these tumors with specific approach applied in some of them. For instance, in case of ocular surface squamous neoplasia complete but gentle surgical excision using a “no-touch” technique is the treatment of choice. Resulting superficial defects can be overlaid with an amniotic membrane.6 On the other hand treatment of conjunctival melanoma is based on certain established principles.7 These extend from complete excision of the lesion in the episcleral plaque with 4mm clinically clear margins and post-surgery adjuvant plaque brachytherapy to the eyelid sparing exenteration, proton beam radiotherapy and systemic chemotherapy.8,9 Part of the ocular surface lesions needs only a periodical observation. In general, benign tumors and choristomas are excised only if there is a cosmetic or functional concern. Malignant tumors generally need complete excision with clear margins and excision edge cryotherapy.7 One of the ways to avoid itching, dry eye symptoms and irritation after surgery of epibulbar tumors is cyclosporin A therapy.
A group of patients after the surgery of the epibulbar tumor was observed in the January-March 2017 period. After surgery cyclosporine A (CsA) treatment was administered once a day. We evaluate results 12months of treatment.
A group of 8 patients, an average age of 43year (23 to 65years). Indications of treatment were: recurrent epibulbar carcinoma-2 patients (25%), dysplastic melanocytic nevus-4 patients (50%), and malignant melanoma of the conjunctiva-1 patient (12.5%), epibulbar MALT lymphoma-1 patient (12.5%).
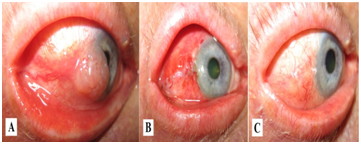
Figure 1 Non-melanocytic epibulbar lesion (carcinoma) before surgery (A), first week after surgery (B) and 3months after surgery and treatment with CsA cationic solution (C).

Figure 2 Melanocytic epibulbar lesion (dysplastic nevocelular melanocytic nevus) before surgery (A), seven weeks after surgery (B) and six months after surgery and treatment with CsA cationic solution.
Patients underwent surgery under local anesthesia. Lesion was removed with a 2mm clinically tumor-free conjunctival part peripheral to the tumor and a thin scleral flap beneath the tumor. During the surgery Mitomycin C was applied topically. The corneal epithelium 2mm anterior to the tumor was not treated with absolute alcohol and we did not use cry therapy as well. After surgery antibiotics and steroids were used for 10days and patients continued therapy with CsA solution once per day use. We evaluated the course of healing for the first 12months after surgery – patients’ dry eye, pain, itching and foreign body feeling after surgery (Table 1). We divided patients into 2 subgroups – patients with melanocytic epibulbar lesions and non-melanocytic epibulbar lesions (Table 1), (Figure 1), (Figure 2).
Treatment\symptoms |
Before surgery |
After surgery |
||||||
|---|---|---|---|---|---|---|---|---|
Melanocytic lesion |
Non-melanocytic lesion |
Melanocytic lesion |
Non-melanocytic lesion |
|||||
|
[#] |
[%] |
[#] |
[%] |
[#] |
[%] |
[#] |
[%] |
Dry Eye |
5 |
62.5 |
3 |
37.5 |
1 |
12.5 |
1 |
12.5 |
Pain |
1 |
12.5 |
1 |
12.5 |
0 |
0 |
1 |
12.5 |
Itching |
4 |
50 |
3 |
37.5 |
0 |
0 |
0 |
0 |
Foreign Body Feeling |
2 |
25 |
1 |
12.5 |
0 |
0 |
0 |
0 |
Table 1 Amplitude in mill volts of the Lead-1 of electrocardiography in sheep
*Significant (P≤0.05); NSNot significant (P>0.05)
We calculated paired t-test with the significance level 0.05 for melanocytic lesions group and non-melanocytic lesions group. The calculation results confirmed that treatment by CsA after surgery of the melanocytic lesions group is considered to be statistically significant (P value equals 0.0351) and the treatment by CsA after surgery of the non-melanocytic lesions group is considered to be not statistically significant (P value equals 0.1027).
Clinical manifestation of the epibulbar tumors is variable. Benign lesions may be stable and asymptomatic and sometimes even undergo spontaneous resolution.9 Other can cause redness, discomfort, local swelling, erythema.7 Ocular surface squamous neoplasia can present even with a course resembling chronic conjunctivitis.10 The most common presentation of conjunctival melanoma is a raised, irregular, unilateral pigmented area, brownish-black in color and most often without other associated symptoms. Less common is the foreign body sensation and pain in the affected eye.11,12
Subjective symptoms following surgical treatment of the epibulbar tumors are also various, including patients’ complaints resembling symptoms of DED. Many published studies also reported origin or escalation of the DED symptoms after other kinds of eye surgery.13–15 Long term artificial tears supplementation is often needed, which typically provides only short-term relief from DED symptoms.16 Cyclosporine A has received increased attention in recent years as a therapeutic agent providing inhibition of the inflammatory responses associated with DED.5,17 Long term treatment is required to achieve the resolution of symptoms such as reduction of inflammatory markers and tear osmolarity, antiapoptotic effects and recovery of reduced conjunctival goblet cell density.17 Cationic emulsion formulation containing 0.1% (1mg/ml) CsA (CsA CE) has been developed and registered in 2015.5 It is a cationic emulsion with a long-lasting presence of the CsA in the tear film.18 In our study we did not confirm in non-melanocytic lesions group to be statistically significant benefit of use after surgical removal of the lesion, but in melanocytic lesions group it was considered to be statistically significant.
The function of tear film in patients with epibulbar tumors is limited. Cyclosporine A is a cyclic polypeptide and belongs to the group of immunosuppressants-calcineurin inhibitors (CNI). CsA specifically affects only the function of T lymphocytes, diminishing the production of cytotoxic T lymphocytes and also antifungal effect was confirmed.19 Application of CsA is another option for treating dry eye syndrome and post-surgery complications of epibulbar tumors, which, in the post-surgery course, is especially important in the treatment of the inflammatory component of the post-surgery response.
The function of tear film in patients with epibulbar tumors is limited. CsA specifically affects only the function of T lymphocytes, diminishing the production of cytotoxic T lymphocytes. Application of CsA is another option for treating dry eye syndrome and post-surgery complications of epibulbar tumors, which, in the post-surgery course, is especially important in the treatment of the inflammatory component of the post-surgery response.
None.
None of the authors has conflict of interest with this submission. All authors have read and approved of the manuscript being submitted.
None of the authors has financial interest related to this study to disclose.
The manuscript does not report the results of an experimental investigation on human subjects.
This article does not include results of experimental investigations on human subjects.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.