Advances in
eISSN: 2377-4290


Recent in-vitro, in-vivo and human studies indicate that dietary meso-zeaxanthin (MZ) may be of benefit in maintaining vision and provides antioxidant and anti-inflammatory support. MZ is a macular carotenoid with additional protective effects such as antioxidant, anti-mutagenic, singlet oxygen quenching and inhibitory effects on specific CYP450 isoenzymes, potential to induce phase II enzymes, chemo-protective and anti-carcinogenic effects. MZ scavenges superoxide, hydroxyl and nitric oxide diphenyl-2-picrylhydrazyl (DPPH) radical, and 2, 2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radicals, inhibits tissue lipid peroxidation and prevents cellular damage. MZ increase the levels of antioxidant enzymes like catalase, superoxide dismutase and glutathione peroxidase. MZ supplementation will provide benefit for maintaining visual performance in health and disease. Recent data strongly support the observation that adequate macular carotenoid supplementation may significantly reduce the risk of macular degeneration and age related macular degeneration mediated by reactive oxygen species. This review will focus on the effect of macular carotenoids and MZ in the context of carotenoids' unique visual health including antioxidant and anti-inflammatory properties.
Keywords: meso-zeaxanthin, macular carotenoids, antioxidant, eye health
MZ, meso-zeaxanthin; L, lutein; RZ, RR-Zeaxanthin; AMD, age-related macular degeneration; MD, macular degeneration; NAFL, non-alcoholic fatty liver; NASH, nonalcoholic steatohepatitis; DPPH, diphenyl-2-picrylhydrazyl; ABTS, 2, 2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); GST, glutathione-s-transferase; UDPGT, uridine diphosphate glucuronyl transferase; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase
Visual function
Healthy eyes and vision are the most important factors for quality of life. The morphological and physiological changes in visual function occur in aging due to several risk factors such as diet, family history and smoking, exposure to environmental affluent and chronic conditions. Visual changes occur at any age. The common morphological and physiological changes include average refractive error, decrease in corneal sensitivity, and transmission of light and volume of the anterior chamber. In addition, pupil size decreases in dim light conditions. With aging, the ocular lens becomes more yellow and absorbs more light, significantly changing the amount and quality of light reaching the retina. The amplitude of accommodation (expressed in diopters), photoreceptor density and visual field size decrease with age as well. The vitreous commonly detaches from the retina after the age of 60. Lipofuscin (aging pigment) accumulates in the retina with age; other retinal cell layers become disordered and visual acuity declines, with age. During aging the ability to adapt to darkness is slowed, the ability to recover visual sensitivity from bright lights and to see rapid flicker worsens and one experiences more glare problems. Over two decades of research suggest the role of macular carotenoids and its correlation with macular pigment to improve visual performance in eye health and disease. Lutein (L), RR-Zeaxanthin (RZ) and Mesozeaxanthin (MZ) carotenoids that have antioxidant properties and cells are protected from oxidative stress and free radicals.
The three carotenoids involved in eye health, especially macular health, are 3R, 3'S-zeaxanthin or meso-zeaxanthin (MZ), 3R, 3'R-zeaxanthin or RR-zeaxanthin (RZ) and lutein (L). These carotenoids are distributed in the macula at epicenter, mid-periphery, and periphery areas, respectively. The protective substance in the macula is macular pigment (MP). Macular pigment (MP) consists of L, RZ and MZ macular carotenoids. Macular pigment acts as an antioxidant in the eye to help maintain healthy vision. It has unique properties such as blue light filtering from different energy sources, and it modulates other photo-physical properties. It is important to consider fortification of xanthophylls as antioxidants for infant and adult eye health. Macular carotenoids augment MP and enhance visual performance in aging. The physiological and morphological changes includes anatomical structure or function in the eye and vision includes changes in co ordinations, wrong field assessment and observing the aids very closely and reading the materials very close to the eyes, unusual observational positions which will interfere with daily activities.
Macular degeneration
Macular degeneration (MD) is one of the causes for blindness. It is the third most important cause of blindness in the world. More people will go blind from retinal degeneration and visual impairment than from cataracts and glaucoma combined. Recent observational and epidemiological data suggest that 60 million people may be affected with blindness. In the U.S it was reported that over 14 million children (aged 12y) and older people developed retinal degeneration and visual health problems with visual acuity of 20/50. It is possible to bring changes in over 11 million Americans to visual acuity of 2040 with refractive correction. The Center for Disease Control (CDC) predicts that number will double by 2020.1 This number increases every year by 1 million in North America. According to Wong and Colleagues,2 age-related macular degeneration (AMD) will affect 119 million people by 2020 and expected to affect 288 million people in 2040.
Age, sex, race, smoking, family history, high blood pressure, prolonged sun exposure and chronic disease conditions are risk factors for macular degeneration. Other risk factors include oxidative stress causing free radicals, high energy UVA, LED blue light sources, such as the sun, television, tablet, cell phones, which exposure may cause photochemical reactions and cellular changes in the macula. Release of free radicals in the macula changes the rate of metabolism due to oxidative stress factors. The release of free radicals from oxidative stress damages the retina, retinal pigment and photo-layers of the retina. Changes due to cellular damage in the retina due to oxidative stress factors leave a brownish pigment (called lipofuscin), which accumulates in Bruch's membrane (BM) and forms drusen. The formation of drusen is the first early sign of dry macular degeneration (DMD). This will lead to loss of field of vision including blurred central vision or a blind spot at any age. The oxidative stress factors that may increase inflammation and cellular changes in the macula causing macular degeneration are seen in Figure 1. The changes in the eye can be determined by an ophthalmologist and the progression of AMD will be categorized as Early AMD (dry AMD) condition, based on several changes and abnormalities including soft drusen, pigment epithelial changes, changes in macula, fatty depots in the retina and thinning of macula, while late AMD (Wet AMD) is characterized by geographic atrophy or neo-vascular AMD and or pigment epithelial detachment.
Macular carotenoids
The biological activity and placement of each of the macular carotenoids – lutein, RR-zeaxanthin and meso-zeaxanthin - in the eye tissue is of interest. The biological role of macular carotenoids includes limiting chromatic aberration at the fovea by filtering out blue light,3 quenching of singlet oxygen or free radicals produced in the retina, protecting the macula from the photo toxicity of blue light4,5 and protecting against photo-oxidation of lysosomal membranes and reducing the risk of AMD.6
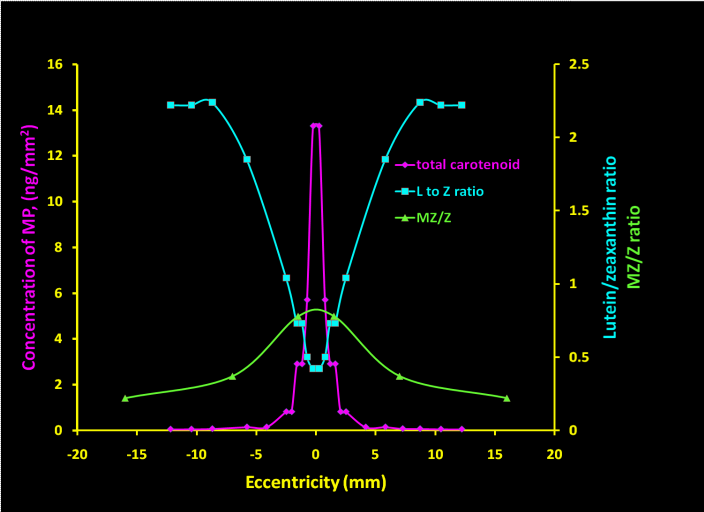
Xanthophylls are yellow or orange pigments in food and plants. Over 600 carotenoids are observed in nature, but not all are useful for health and disease condition (Table 1). The carotenoids in the retina are lutein (L), RR-zeaxanthin (RZ) and meso-zeaxanthin (MZ). Meso-zeaxanthin (MZ, Figure 2) comprises 33% of the total carotenoid content in the macula. RR-zeaxanthin (RZ) is concentrated in the macular region, whereas lutein (L) is dispersed throughout the entire retina. Meso-zeaxanthin concentration is greatest at the peak and decreases rapidly away from the peak.7 Substantial quantities of carotenoids are also present in the infant retina. Recent research also suggests that carotenoids from food sources together may play a crucial role in retinal health and visual function in infants8−11 and abnormal carotenoids may lead to oxidative stress and inflammation).12−15 Carotenoid levels between individuals vary with advancing age.16 Figure 1 also shows how macular carotenoids prevent MD and progression of early and late AMD risk.
Carotenoid |
Ave. plasma concentration (.tmol/L) |
Retinal concentration |
Structure |
||
|---|---|---|---|---|---|
Content (pmoles) |
Total (pmoles) |
O,` ' |
|||
Lutein |
0.25 |
Central: 17 |
59 |
58% |
|
Medial: 20 |
|||||
Outer: 22 |
|||||
Mesa- zeaxanthin |
Trace |
Central: 10 |
15 |
15% |
|
Medial: 3 |
|||||
Outer: 2 |
|||||
RR- zeaxanthin |
0.06 |
Central: 12 |
28 |
27% |
|
Medial: 9 |
|||||
Outer: 7 |
|||||
Table 1 Macular carotenoids structure and concentration distribution in plasma and retina48
Dietary intake of macular carotenoids
The dietary data from National Health and Nutrition Examination Survey (NHANES) 2003-2004 reported that the average intakes of lutein and zeaxanthin isomers from dietary sources are in the range of 1 to 2 mg/day (approximately 0.01 to 0.03 mg/kg body weight/day) and it was observed that their intakes are low compared with the average requirement of 10 mg L/d to maintain vision health. Studies reported efficacy in AMD is at a dose range of 6-40 mg L/day. In general, the ratio of lutein to zeaxanthin isomers in natural dietary sources is approximately 5:1.17 Numerous studies have demonstrated that increased dietary intake of lutein and zeaxanthin isomers is associated with increased macular pigment density (MPOD) in healthy adults.17,18 A negative correlation was observed with dietary intake of macular carotenoids and with the risk of developing ocular diseases such as early and late age-related macular degeneration (AMD) and cataracts.19−21
The major dietary sources of carotenoids (Lutein and Zeaxanthin) include yellow and orange fruits and vegetables and dark green leafy vegetables, such as spinach, kale, kiwi and yellow pepper, as well as eggs. The major dietary sources of MZ are shrimp shells, turtle fat 21 species of fish skin, as well as eggs in California and Mexico. MZ absorption may be increased by cooking these dietary sources in a small quantity of oil. MZ belongs to xanthophyll class fortified in chicken feed in Mexico for the last 10 years. Egg consumption in Mexico is approximately one egg/person/day and contributes to dietary intake of MZ in this population.17 further epidemiological data and observational studies are required to explore the intakes of dietary MZ in different population groups.
Blue light and macular carotenoids
The macular carotenoids are very effect in absorbing light of different wavelengths between 400-540nm. These carotenoids protect the retina and underlying macular layers (rods and cones) from ultraviolet A and high energy blue light from LED sources. The energy from different sources such as ultraviolet A and high energy blue light from LED may cause free radicals, develop oxidative stress and cause retinal damage and visual impairment. Dietary carotenoids (L, RZ and MZ) may filter the damaging light and improve macular pigment optical density (MPOD). There was a significant correlation of MPOD and macular carotenoids. This will reduce and prevent the damage to the cells of the macula. MPOD varies greatly from one individual to another and declines with age. In addition to the normal age-related reduction of macular pigment, MPOD is related to genetic make-up, prior history of light exposure, diet and lifestyle factors.
The absorption spectrums of macular carotenoids are 400–540 nm, peaking at approximately 460 nm. Carotenoids attenuate and modulate the amount of blue light reaching the photoreceptors and prevent photo-damage. There was a direct relation of the higher the density of macular pigment the greater the exposure to the amount of blue-light filtering. The antioxidant functions of macular carotenoids may protect against dry and wet AMD. This review summarizes the current concepts, perspectives and new insights of the macular carotenoid MZ in health and disease.
Bioavailability
Bioavailability is defined as the extent and rate at which the active moiety enters systemic circulation, thereby accessing the site of action. MZ bioavailability was reported in volunteers (ten men and nine women) who received one capsule of 10.8 mg L, 1.2 mg RZ and 8.0 mg MZ/d. Blood was taken at baseline, day 10 and day 22. Concentrations of MZ at day 22 were two folds higher in women than men. A significant variation exists in the absorption of carotenoids and gender response.22 Although the uptake of L into plasma appeared to be slightly depressed by the presence of MZ, it is difficult to draw a conclusion based on this study that MZ inhibit L absorption. Further, recent studies suggest no inhibition of L or other carotenoids by MZ in intervention studies reported by Nolan et al. and his research group. MZ supplementation increased MZ levels in blood and improved visual performance by increasing MPOD (Table 2). In another study, a significant correlation of MPOD across its spatial profile was observed after supplementation with a formulation containing high doses of MZ (17 mg) in combination with L and RZ compared to L/RZ supplementation alone. There was an improvement in contrast sensitivity with the supplementation of macular carotenoids.23 Bioavailability of an ingredient is largely determined by the properties of the dosage form and quality of the product, which depend partly on its design and manufacturing process. Further dose response and tissue absorption studies are required.
|
Author |
Condition |
Gender |
Age |
Study Design |
N |
Study Duration |
L(mg) |
RRZ(mg) |
MZ(mg) |
Dosage Forms |
Outcome Measures |
Interpretation |
Safety |
|
Bone et al.26 |
Healthy Subjects |
8M /2F |
30.5 ± 10.9 y |
Parallel; RPC |
Supplement: 10 |
120 d |
5.5 |
1.4 |
14.9 |
Softgels |
Serum macular carotenoids, MPOD |
• Presence of all three carotenoids in actives supplementation. |
No adverse events reported |
|
• MPOD, measured at 460 nm, rose at average rate of 0.59 +/- 0.79 milli-absorbance unit/day in supplement group. |
|||||||||||||
|
5M/ 4 F |
22.1 ± 3.6 y |
Placebo: 9 |
0 |
0 |
0 |
• MZ absorbed into serum following ingestion. Supplement group significantly different from placebo group for whom the average rate was -0.17 +/- 0.42 milli-absorbance units/day. |
|||||||
|
Thurnham et al.22 |
Healthy Subjects |
9F |
27 ± 7 y |
Open label, non randomized |
19 |
22 d [baseline, day 10 and day 22] |
10.8 |
1.2 |
8 |
Softgels |
Serum macular carotenoids |
• Plasma concentrations per mg dose at day 22 suggested that RR-zeaxanthin (0.088 μmol/l per mg) was about 50 % more actively retained by the body than lutein (0.056 μmol/l per mg) (difference not significant in women) and 2.5-3.0x more than MZ (0.026 μmol/l per mg). |
No adverse events reported |
|
• MZ concentrations at day 22 were 2.5x higher in women than men. Plasma responses from lutein and RR-zeaxanthin in Lutein Plus lower than literature values. Plasma uptake appeared to be slightly depressed by presence of MZ. |
|||||||||||||
|
10M, smokers |
34 ± 9 y |
• Plasma concentrations of beta-carotene were depressed by about 50% at day 10 and about 35% at day 22. |
|||||||||||
|
Connolly et al.30 |
Normal |
5 M or F |
18-60 y |
Open label; non randomized |
10 |
56 d |
3.7 |
0.8 |
7.3 |
Capsule |
Serum macular carotenoids, spatial profile of MPOD |
• Significant increase in serum concentrations of MZ and L after 2 weeks supplementation (p < 0.05). |
No adverse events reported |
|
• Baseline serum carotenoid analysis detected small peak eluting at same time as MZ in all subjects, with a mean +/- SD of 0.02 +/- 0.01 μmol/L. |
|||||||||||||
|
Early AMD |
5 |
• Significant increases in MPOD at 0.25 degrees, 0.5 degrees and 1 degree, and for average MPOD across spatial profile after two weeks supplementation (p < 0.05, for all). |
|||||||||||
|
Early AMD |
5 M or F |
• Four subjects (one normal and three AMD) who had an atypical MPOD spatial profile (i.e., central dip) at baseline had more typical MPOD spatial profile (i.e., highest MPOD at center) after 8 weeks supplementation |
|||||||||||
|
Connolly et al.29 |
Healthy Subjects |
M/F |
18 - 61 y |
RPC |
intervention, I group :22 or placebo, P group :22 |
6 mos [Bl, 3 mos and 6 mos) |
5.9 |
1.2 |
10.6 |
Capsule |
Serum macular carotenoids, spatial profile of MP optical density |
• Subjects supplemented with MZ, L and Z exhibited significant increases in serum concentrations of these carotenoids and subsequent increase in central MPOD. |
Pathology analysis suggested no adverse clinical implications of consuming these carotenoids |
|
Nolan et al.31 |
Healthy Subjects with atypical MP spatial profiles |
M/F |
18 -60 y |
Randomized Trial |
Group 1: (n = 10) |
8 wks [baseline, 4 wks and 8 wks] |
10 |
2 |
0 |
Softgels |
MP at 0.25°, 0.5°, 1°, 1.75° and 3° |
• No statistically significant increase in MP at any eccentricity in Group 1 (p > 0.05, for all eccentricities). |
No adverse events reported |
|
• Trend towards increase in MP at all eccentricities in Group 2, with significant increase at 0.25° and 0.50° (p = 0.000 and p = 0.016, respectively). |
|||||||||||||
|
Group 2: (n = 10) |
10 |
2 |
10 |
• Statistically significant increase in MP at 0.25° in Group 3 (p = 0.005), but at no other eccentricity (p > 0.05, for all other). |
|||||||||
|
Group 3: (n = 10) |
3 |
2 |
17 |
• Typical central peak of MP can be realized in subjects with atypical spatial profiles following supplementation with preparation containing all three macular carotenoids, but not with a supplement lacking MZ. |
|||||||||
|
Meagher et al.28 |
Normal |
54 total Normal 27, AMD 27 20 M, 34 F |
60 ± 10 y |
Randomized Trial Dobule blind |
Group I: 21a |
8 weeks [baseline, 4 weeks and 8 weeks] |
20 |
2 |
0 |
Softgels |
Serum macular carotenoids |
• Response as average of 4- and 8-week concentrations (saturation plateau). |
|
|
• Serum L increased significantly in Group 1 (0•036 μmol/l per mg (269 %); P< 0•001) and Group 2 (0•079 μmol/l per mg (340 %); P< 0•001), with no significant change in Group 3 (0•006 μmol/l per mg (7 %); P= 0•466). |
|||||||||||||
|
AMD |
66 ± 7 y |
Group 2: 20b |
10 |
2 |
10 |
• Serum RRZ increased significantly in Group 1 (0•037 μmol/l per mg (69 %); P= 0•001) and Group 2 (0•015 μmol/l per mg (75 %); P< 0•001), with no significant change in Group 3 ( − 0•0002 μmol/l per mg ( − 6 %); P= 0•384). |
|||||||
|
Group: 13c |
3 |
2 |
17 |
• Serum MZ increased significantly in Group 1 (0•0094 μmol/l (absolute value); P= 0•015), Group 2 (0•005 μmol/l per mg; P< 0•001) and Group 3 (0•004 μmol/l per mg; P< 0•001). |
|||||||||
|
Loughman et al.31 |
Normal Subjects |
19 M, 17 F |
51 ± 13 y |
Single-masked placebo-controlled study |
Group I: 11a |
6mos [Baseline, 3 mos and 6 mos] |
20 |
2 |
0 |
Softgels |
MPOD and visual performance |
• At 3 and 6 months, statistically significant increase in MPOD at all eccentricities (other than the most peripheral 3° location) in group 2 (P < 0.05 for all), whereas no significant increase in MPOD was demonstrable at any eccentricity for subjects in groups 1 and 3. |
No adverse events reported |
|
• Statistically significant improvements in visual performance measures including visual acuity and contrast sensitivity with and without glare were observed for group 2 only. |
|||||||||||||
|
Group II: 11b |
10 |
2 |
10 |
• Only mesopic contrast sensitivity at one spatial frequency improved significantly by 6 months (P < 0.05) for group 1. |
|||||||||
|
Group III (P): 10c |
0 |
0 |
0 |
• No improvements in any parameters of visual performance were observed for subjects supplemented with placebo (P > 0.05 for all). |
|||||||||
|
Loughman et al.27 |
AMD |
M/F |
18-60 y |
Randomized trial |
Group I: 23 |
36 mos |
20 |
2 |
Softgels |
MPOD,visual function-visual acuity,letter contast sensitivity |
• A statistically signficant increase in MPOD observed in all measured eccentricities with exception in group I at 1.7. |
No adverse events reported |
|
|
• In group I, statistically significant improvements in letter contrast sensitivity were only seen at 1.5 cpd. |
|||||||||||||
|
• In Group 2, statistically significant improvements in letter CS were seen at all spatial frequencies except at 2.4 cpd. |
|||||||||||||
|
• In Group 3, statistical significant improvement in letter CS was seen at 6 cpd, 9cpd, and 15 cpd. |
|||||||||||||
|
Group II: 24 |
10 |
2 |
10 |
• 31 of 46 subjects exhibited no change in AMD grade over the three years. |
|||||||||
|
Group III: 20 |
3 |
2 |
17 |
• No subject exhibited beyond stage 8 on the AREDSII step severity scale. |
|||||||||
|
Sabour-Pickett et al.23 |
Early age-related macular degeneration |
Randomized Trial |
Group I: 17 |
12 mos |
20 |
2 |
Softgels |
MPOD,visual function-visual acuity,letter contast sensitivity |
• Statistically significant increase in MPOD at all measured eccentricities in Group 2 (P ≤ 0.005) and in Group 3 (P < 0.05, for all), but only at 1.75° in Group 1 (P = 0.018). |
No adverse events reported |
|||
|
Group : 21 |
10 |
2 |
10 |
• Statistically significant (P < 0.05) improvements in letter contrast sensitivity at all spatial frequencies (except 1.2 cycles per degree) in Group 3, and at low spatial frequencies in Groups 1 and 2. |
|||||||||
|
Group : 14 |
3 |
2 |
17 |
Table 2 Summary of MZ human clinical trials
L, lutein; RRZ, RR-zeaxanthin; MZ, meso-zeaxanthin; R, randomized; DB, double blind; SB, single blind; PC, placebo controlled a group 1; b Group 2; c Group 3; *No MZ was present in the serum baseline.
Biological functions of meso-zeaxanthin
MZ has substantial antioxidant, anti-inflammation and immune functions. Although MZ is primarily associated with visual function and its protective role in eye health, recent studies show a number of additional properties of the macular carotenoid which need further attention, such as anti-cancer/tumors potential, liver health protection and healthy metabolism effects.
Eye health: MZ is able to protect against chronic and cumulative eye damage through its capacity to filter the most energetic and potentially damaging wavelengths of visible light and to neutralize free radicals produced by oxidative stress.24 In-vitro and in-vivo studies of this carotenoid showed that it has significant antioxidant potential.25 Table 2 provides a summary of MZ supplementation studies in combination with L/RZ. Human clinical studies demonstrate MZ's protective role for eye health and defense against AMD.26−31 Overall, in these studies, MZ was observed in blood after supplementation. Doses used in these studies vary from 8 mg to 17 mg/d. Improvements in visual performance with MZ supplementation was observed in human clinical trials (Table 2). MZ is absorbed into the serum following supplementation in all the clinical studies. The data indicate that a supplement containing predominantly MZ is generally effective at raising macular pigment density, and may be a useful addition to the defenses against AMD and to support eye health in general.
MZ as a strong antioxidant
Effect of MZ on cytokines and oxidative stress: Release of pro-inflammatory cytokines and oxidative stress in the liver activate the resident immune cells of the liver, such as Kupffer cells and stellate cells, which mediate liver injury. Carotenoids are recognized to be free radical scavengers as well as chain breaking antioxidants and can scavenge reactive oxygen species (ROS) near the membrane surface, while the polyene chain would inhibit the free radical chain reaction into the membrane32 Oxidative stress enhances free radicals; β-oxidation of free fatty acids produces hydrogen peroxide in the liver thus causing inflammation and increasing reactive oxygen species and leading to liver necrosis and liver disease conditions.33
MZ can be considered a chemo-preventive agent by virtue of its protective properties like antioxidant, anti-mutagenic and singlet oxygen quenching effects, inhibitory effects on specific CYP450 isoenzymes, potential to induce phase II enzymes and anti-carcinogenic effects.34 MZ has substantial antioxidant activity. The anti-carcinogenic activity of this carotenoid can also be due to the quenching of oxygen radicals produced during the promotion stage of carcinogenesis.34,35 Preliminary evidence suggest that supplementation of MZ was found to scavenge superoxide and hydroxyl radicals and inhibited in-vitro lipid peroxidation25 Oral administration of MZ inhibited superoxide radicals generated in macrophages by 25.2%, 50.1% and 67.2% at three different doses of low (50), moderate (100) and high (250 mg/kg bw), respectively. After one month MZ supplementation in mice, antioxidative enzymes such as catalase, superoxide dismutase, glutathione and glutathione reductase levels in blood and liver significantly increased. Antioxidative enzyme levels of glutathione peroxidase and glutathione-S-transferase were also found to be increased in the liver in a dose dependent manner.25 These results suggest antioxidative properties of MZ, and further long term human studies are required to explore its role in health and disease conditions.
Hepatic fibrosis is one of the liver disease during which excess connective tissue will build in liver and up-regulate cytochromes. CYP2E1 is one of the cytochrome gene proteins involved in oxidative stress process and CYP2E1upregulates collagen I in rat hepatic stellate cells.36 This process leads to the depletion of antioxidative enzymes and inhibits the antioxidant system. The gene protein cytochrome CYP2E1 up regulates by high fat/low-carbohydrate diets.37 Over-expressions of cytochrome gene proteins has a significant role in the inhibition of antioxidant system process. Antioxidants play a role in the prevention of conditions associated with liver health and disease.37−39 Preliminary evidence suggests that oral administration of MZ at different doses significantly increases tumor latency period. In 3-methylcholanthrene (3-MC) control group, animals started developing sarcoma in week 6. However, animals treated with 3-MC and MZ (50 and 250 mg/kg bw) started developing sarcoma only on 15th and 18th week, respectively. Survival of tumor-bearing mice was significantly increased by MZ treatment. Animals in 3-MC control group started dying due to tumor burden from the 8th week. All animals treated with MZ (50 and 250 mg/kg bw) were found to be alive even after 16 and 20 weeks, respectively. Oral administration of MZ inhibited different CYP450 isoenzymes like CYP1A1 (PROD), CYP1A2 (MROD), and CYP2B1/2 (EROD), which are involved in carcinogen metabolism in a dose-dependent manner. Moreover, levels of phase II enzymes like UDP-glucuronyl transferase and glutathione-S-transferase, which are involved in detoxification of carcinogens, were significantly increased by MZ treatment. Results indicated that the mode of action of MZ may be through inhibition of carcinogen activation coupled with enhancement of the detoxification process. MZ may also inhibit promotion phases of carcinogenesis by its antioxidant activity.40 CYP inhibition is an important consideration for the development of novel therapeutic agents. Clinically important mechanism-based CYP inhibitors include anti-bacterial, anticancer agents, anti-HIV agents, anti-hypertensives, sex steroids and their receptor modulators.41
Effect of MZ on antioxidant enzymes
Oxidative stress plays a central role in liver disease pathogenesis and progression, and the use of antioxidants has been proposed as therapeutic agents. MZ is one of the macular carotenoids and acts as an antioxidant to protect against cellular damage from free radicals. Recent reports suggest that oxidative stress markers, tissue lipid peroxidation, conjugated dienes and tissue hydroperoxides were enhanced with paracetamol treatment. In an animal study, paracetamol was compared to a control group, and MZ supplementation to these animals improved the level of antioxidative enzymes. In an alcoholic induced animal model study, the levels of antioxidant properties and antioxidant enzymes improved with MZ supplementation (glutathione and antioxidant enzymes, superoxide dismutase, catalase and glutathione peroxidase) in liver tissue. The hepato-protective potential of MZ was observed in these animals.42 These results suggest a role of MZ as an antioxidant. Recent human studies also confirmed MZ supplementation will protect liver and renal functions including maintenance of normal lipid profile and hematological indicators.29 Further long term humans studies are required to confirm these findings.
Potential mechanism of action
Visual performance
MZ is required for macular pigmentation for healthy eye function. MZ may reduce the production of reactive oxygen species (ROS), and MZ inhibit speroxidation and reduces oxidative injury. MZ also filters blue light in the macula.27
Antioxidant activity
The mode of action of MZ may be through enhancement of the detoxification process, reducing enzymes such as Serum glutamic oxaloacetic transaminase (SGOT) and Serum glutamic-pyruvic transaminase (SGPT). Toxin blockade at the membrane level inhibit membrane peroxidation. MZ has been shown to reduce hepatotoxins in the body and to stimulate glutathione, a powerful antioxidant that can help protect liver cells, support detoxification enzymes more effectively and protect the liver from toxins, including certain drugs such as acetaminophen (Tylenol), which can cause liver damage in high doses, have antioxidant and anti-inflammatory properties, and may help the liver repair itself by growing new cells.25,40,42 In preclinical studies, one month MZ supplementation increases antioxidant enzymes in mice.43,44 Laboratory studies show that MZ scavenges superoxide, hydroxyl, nitric oxide and DPPH and ABTS radicals, as well as inhibits tissue lipid peroxidation in-vitro in a concentration dependent manner.25
The possible anti-carcinogenic activity of MZ and its effect on phase I carcinogen metabolizing enzymes was studied. The result indicated that MZ could significantly inhibit different CYP450 isoenzymes (CYP1A1, CYP1A2, and CYP2B1/2), which are involved in the activation of many known chemical carcinogens. This inhibitory effect could be one of the mechanisms of action of MZ against chemical carcinogenesis. Another major mechanism of protection against chemical carcinogenesis is mediated by the induction of enzymes involved in the detoxification of chemical carcinogens. Phase II enzymes such as glutathione-S-transferase (GST) and uridine diphosphate glucuronyltransferase (UDPGT) are the major enzymes involved in the detoxification process. Transcriptional control of the expression of phase II enzymes is mediated through the antioxidant response element (ARE) found in the regulatory regions of their genes. The binding of transcription factor Nrf2 to ARE in response to treatment with certain phyto chemicals appears to be essential for the induction of prototypical phase II enzymes.45
Safety/Toxicity
MZ is nontoxic and generally regarded as safe. The European Food Safety Authority (EFSA) concludes MZ is a food constituent.46 Standard protocols of toxicity were used to study the acute and chronic toxicity in animals. There were no adverse events in animal and human studies and no-observed-adverse-effect-level (NOAEL) of MZ was >200mg/kg/day in animal studies. No cytotoxicity, no mutagenesis and no geno toxicity were observed with MZ administration.47−50 In humans, no adverse events were reported in any of the human clinical trials (Table 2).
MZ, one of the three macular carotenoids, improves visual function and has been shown to be very effective in the early stage of AMD. It is a very strong antioxidant to protect against oxidative stress. In addition to supplementation of macular carotenoids, smoking cessation diet, regular physical activity and protective eye wear such as sunglasses and wide brimmed hats may help reduce the risk of AMD, and regular eye examinations can result in the early detection of early and late age related macular degeneration. The preservation of vision at any age and quality of life are related to a healthy diet including the macular carotenoids, physical exercise and the reduction of exposure time of eyes to high energy sources. Early macular carotenoid interventions that are geared to improve visual performance in healthy and high risk populations are imperative.
None.
Employee, Omni Active Health Technologies Inc.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.