eISSN: 2378-3176


Research Article Volume 8 Issue 4
1Jimma University health sciences institute, Jimma Ethiopia
2Hawassa University college of Medicine and health sciences, Hawassa Ethiopia
Correspondence: Deresse Daka: Hawassa University, College of medicine and heath sciences, Hawassa Ethiopia, Tel +251911968912
Received: May 04, 2020 | Published: July 23, 2020
Citation: Mohammed A, Beyene G, Teshager L, et al. Urinary pathogenic bacterial profile, antibiogram of isolates and associated risk factors among diabetic patients in Hawassa town, southern Ethiopia: a cross-sectional study. Urol Nephrol Open Access J. 2020;8(4):84-91. DOI: 10.15406/unoaj.2020.08.00282
Urinary tract infection (UTI) is the commonest bacterial infectious disease in community practice with a high rate of morbidity and financial cost. It has been estimated that 150 million people were infected with UTI per annum worldwide which costing global economy more than 6 billion US dollar. In humans, urinary tract is the second commonest site after the respiratory tract, for bacterial infection. Two hundreds forty seven diabetic patients were investigated for UTI using 5 to 10ml midstream urine sample. A loop full urine sample was inoculated on cysteine lactose electrolyte deficient (CLED) agar, MacConkey agar and Blood agar plates. Antimicrobial susceptibility test was done using Clinical and Laboratory Standards Institute (CLSI) for all patients. Age, sex, resident, marital status and other factors were used as exposure factor of the cross sectional study. The aim of this study was to assess etiology, risk factors and drug sensitivity pattern of uropathogenic bacteria isolated from diabetic patients. The overall prevalence of significant bacteriuria was 26(10.5%). Significant bacteriuria was significantly associated with age and body mass index. The predominant bacteria isolate was E. coli 12(46.2%) followed by Coagulase negative staphylococcus 7(26.9%).Gram negative bacteria showed high rate of sensitivity (94.1%) to Nitrofurantoin and Norfloxacine. Gram positive bacteria showed 100% sensitive for Amoxacillin-Clavunic acid. Multidrug resistance to two or more drug was observed in 19(73.1%) of bacteria isolates. The overall prevalence of significant UTI in diabetic patients was 10.5%. The most frequently observed organisms were E. coli, CONs, K. pneumoniae, K. oxytoca and S. aureus. Sex, age, BMI, occupational status such as house wife was statistically significant. Gram negative bacteria isolates were the most common antibiotic resistant bacteria isolates from UTI patients.
Keywords: diabetes, UTI, uropathogens, antimicrobial susceptibility
UTI, urinary tract infection; US, United States; BMI, body mass index; CONs, Coagulase negative Staphylococcus; HUCSH, Hawassa university college of Medicine and health sciences
Urinary tract infection (UTI) is the commonest bacterial infectious disease in community practice with a high rate of morbidity and financial cost. It has been estimated that 150 million people were infected with UTI per annum worldwide which costing global economy more than 6 billion US dollar.1 In humans, urinary tract is the second commonest site after the respiratory tract, for bacterial infection.2 Many different microorganisms can cause UTIs though the most common pathogens causing are Escherichia coli and other Enterobacteriacae, which accounts approximately 75% of the isolates.3 Gram-positive bacteria such as Enterococcus spp. and Staphylococcus spp. can also cause UTIs.4 Over time, patients with diabetes may develop cystopathy, nephropathy, and renal papillary necrosis, complications that predispose them to UTIs. Diabetes mellitus increased risk for UTI.5 Higher percentage of resistance to the most commonly prescribed antimicrobial such as Ampicillin, Tetracycline, and Trimethoprim-sulphamethoxazole are reported in isolates from diabetic patients.4,6,7 History of previous UTI, previous antibiotic treatment, recent sexual behavior, type II diabetes, inadequate glycemic control, and duration of DM have strong association with significant bacteriuria in both symptomatic and asymptomatic diabetic patients.4 Since prevalence and type of etiological agent as well as drug susceptibility pattern may vary from time to time or from place to place, it is important to make periodic evaluation that leads to get updated information, however, there is little study conducted on bacterial etiologies, risk factors and antibiotic-resistance patterns in the Southern part of Ethiopia. Therefore this study was undertaken to identify the etiologic agents of UTI, risk factors and their antibiotic resistance patterns among diabetic patients at Hawassa University Referral Hospital, Southern Ethiopia.
Study design and area
A cross-sectional study was conducted from March to May 2018 at Hawassa University Referral Hospital, Southern Ethiopia. The hospital is a tertiary level teaching Hospital that provides health services to over six million inhabitants in southern Ethiopia and it is located 275km south from the capital city, Addis Ababa.
Specimen collection and processing
Five to 10ml of midstream urine specimen was collected from each diabetic patient and labeled with a unique sample number. The specimens were processed within an hour of collection.8
Culturing and identification of isolates
A loop full of urine was inoculated on cysteine lactose electrolyte deficient (CLED) agar, MacConkey, and Blood agar plates (Oxoid, Ltd., Basingstoke, Hampshire, England) by using a sterile calibrated wire loop with a volume of 0.001ml after the specimen was mixed. The plates were incubated aerobically at 35-37oc for 24 hour and the outcome was judged as significant/ non-significant growth, or contaminated (discarded). Urine culture plates showing ≥105 colony-forming units (CFU)/ml of single bacterial species were considered as significant bacteriuria.9 Gram reaction of the organisms, microscopic appearance and colony characteristics were the presumptive identification criteria. Indole production, citrate utilization, H2S production, gas production, urea hydrolysis, lysine decarboxylation, lactose fermentation and motility were used for further identification of gram negative bacteria. Coagulase, catalase, and mannitol fermentation test were used for further identification of gram positive bacteria.8
Antimicrobial susceptibility tests
Antimicrobial susceptibility pattern was performed for all positive isolates using the standardized Kirby Bauer disc diffusion technique according to the criteria of the Clinical and Laboratory Standards Institute (CLSI).10 Then Antibiotic impregnated discs Ampicillin (AMP, 10μg), Trimethoprim-sulfamethoxazole (SXT, 75μg), Amoxicillin-Clavulanic acid (AMC, 30μg), Gentamycin (CN, 10μg), Ceftriaxone (CRO, 30μg), Nitrofurantoin (F, 300µg), Norfloxacin (NOR, 10µg), Nalidixic acid (NA, 30μg), Tetracycline (TE, 30μg) Ciprofloxacin (CIP, 5μg) and Penicillin (P, 10 IU) were placed onto the surface of. Mueller-Hinton agar. Standard strains of E. coli ATCC 25922 and S. aureus ATCC 25923 were used to check the quality of culture and as a control for antimicrobial susceptibility testing.
Data analysis
Data were cleaned, enter into a computer and statistical analysis were performed by using SPSS v.21 statistical software package. Logistic regression analysis was done to determine the association between independent and dependent variables. All independent variables with a p-value less than or equal to 0.2 in the bivariate analysis were included in the multivariate logistic regression model to identify variables which were associated independently. Odds ratio (OR) within 95% confident interval (CI) was calculated to measure the strength of association, and p-value <0.05 was considered statistically significant. Hosmer-Lemeshow and multi - collinearity tests were done to assess the goodness-of-fit of the model and the presence of collinearity of the variance, respectively.
Socio- demographic characteristics
A total of 247 diabetic patients were investigated for UTI. Majority of the participants were male 145 (58.7%) and the remaining 102(41.3%) were female with male to female ratio of 1.42: 1. The mean age of study participants were 45.0+13.7years (range, 18-79 years). Majority of study participants were from urban area 192(77.7%), married 218 (88.3%) and literate 196 (79.4%). The occupational status of a study participants, 82(33.2%) were merchant and 57(23.1%) were house wife (Table 1).
|
Variable |
Categories |
Frequency |
% |
|
|
Age |
||||
|
18-39 |
84 |
34 |
||
|
40-59 |
118 |
47.8 |
||
|
>=60 |
45 |
18.2 |
||
|
Sex |
||||
|
Male |
145 |
58.7 |
||
|
Female |
102 |
41.3 |
||
|
Resident |
||||
|
Urban |
192 |
77.7 |
||
|
Rural |
55 |
22.3 |
||
|
Marital status |
||||
|
Married |
218 |
88.3 |
||
|
Unmarried* |
29 |
11.7 |
||
|
Education |
||||
|
Illiterate |
51 |
20.6 |
||
|
Literate** |
196 |
79.4 |
||
|
Occupation |
||||
|
Farmer |
41 |
16.6 |
||
|
Merchant |
82 |
33.2 |
||
|
House wife |
57 |
23.1 |
||
|
Civil servant |
47 |
19 |
||
|
Others*** |
20 |
8.1 |
||
Table 1 Frequency of Socio-demographic variables of diabetic patients diagnosed for UTIs from March- May, 2018 at HUCSH, Hawassa, and South Ethiopia
*Single, Divorced, Widowed; **Primary school and above; ***Student, Daily labor
Clinical characteristics
Among 247 study participants, 198(80.2%) patients had no symptoms of UTI and the remaining 49(19.8%) presented with symptoms of UTI. Majority of participants were type II DM 202(81.1%). Duration of DM less than 5 years observed in 150(60.7%) of participants. History of previous UTI and catheterization were found in 18(7.3%) and 7(2.8%) of study participants, respectively. Body mass index <25kg/m2, 25-29.9kg/m2 and >=30kg/m2 were found in 147(59.5%), 81(32.8%), and 19(7.7%) of study participants, respectively (Table 2).
|
Variable |
Categories |
Frequency |
% |
|
Symptoms |
|||
|
Symptomatic |
49 |
19.8 |
|
|
Asymptomatic |
198 |
80.2 |
|
|
Type of DM |
|||
|
Type I |
45 |
18.2 |
|
|
Type II |
202 |
81.8 |
|
|
Duration of DM |
|||
|
<5 year |
150 |
60.7 |
|
|
>= year |
97 |
39.3 |
|
|
FBS(mg/dl)* |
|||
|
<126 |
79 |
32 |
|
|
>=126 |
168 |
68 |
|
|
Medication for DM |
|||
|
Tablet |
150 |
60.7 |
|
|
Insulin |
74 |
30 |
|
|
Both |
23 |
9.3 |
|
|
Co-morbidity** |
|||
|
Yes |
70 |
28.3 |
|
|
No |
177 |
71.7 |
|
|
Previous UTI |
|||
|
Yes |
18 |
7.3 |
|
|
No |
229 |
92.7 |
|
|
History of catheterization |
|||
|
Yes |
7 |
2.8 |
|
|
No |
240 |
97.2 |
|
|
BMI(kg/m2)*** |
|||
|
<25 |
147 |
59.5 |
|
|
25-29.9 |
81 |
32.8 |
|
|
>=30 |
19 |
7.7 |
|
Table 2 Frequency of Clinical variables of diabetic patients from March to May, 2018 at HUCSH, Hawassa, South Ethiopia
*Fasting blood sugar; **Hypertension, Blindness***Body mass index
Significant bacteria isolates
The overall bacterial isolates of the current study was 10.5%. Out of the 26 bacteria isolated from the samples, 18(69.2%) were from female and the remaining were from male participants. Among the 26 isolate 17(65.4%) were gram negative bacteria and 9(34.6%) were gram positive bacteria. Six different bacteria species were isolated from study participants. The predominant bacteria isolates were E. coli 12(46.2%), Coagulase negative staphylococcus (CONs) 7(26.9%), S. aureus 2(7.7%), K. pneumonia 2(7.7%) and K. oxytoca 2(7.7%) (Figure 1).
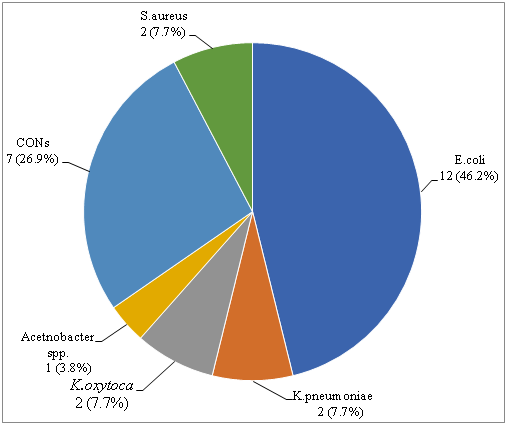
Figure 1 Distribution of bacteria isolated from diabetic patients from March- May, 2018 at HUCSH, Hawassa, South Ethiopia.
Risk factors
In bivariate logistic regression analysis female sex, age range of 40-59 years, house wife occupation, symptoms of UTI, duration of DM>= 5 years, insulin medication, history of previous UTI and BMI >=30kg/m2 were met cutoff criteria of P< 0.25 and the candidate variables for multiple logistic regression analysis. In multiple logistic regression analysis the risk variables, age range 40-59 years (AOR=0.19; 95%CI=0.05-0.65; p<0.01) and BMI>=30kg/m2 (AOR=14.44; 95%CI=3.55-58.77; p<0.01) were found to be statistically significant associated with bacteriuria. Age range of 40-59 years were 80% less likely develop significant bacteriuria than age range of 18-39 years (AOR=0.19; 95%CI=0.05-0.65; p<0.01). DM patients with BMI>=30kg/m2 (AOR=11.94; 95%CI=3.92-47.30; p<0.01) had higher odd ratio compared with those BMI<25kg/m2. However, sex, occupation, symptoms of UTI, duration of DM, medication for DM and history of previous UTI were not found to be significantly associated with significant bacteriuria (p>0.05) (Table 3).
|
Characteristics |
Significant bacteriuria |
No significant bacteriuria |
COR (95%CI) Crude odds ratio |
p-value |
AOR (95%CI) Adjusted odds ratio |
p-value |
|
Age |
||||||
|
18-39 |
13 (15.5) |
71 (84.5) |
1 |
1 |
||
|
40-59 |
8 (6.8) |
110 (93.2) |
0.40 (0.16-1.01) |
0.05 |
0.19 (0.05-0.65) |
<0.01 |
|
>=60 |
5 (11.1) |
40 (88.9) |
0.68 (0.23-2.06) |
0.72 (0.18-2.87) |
||
|
Sex |
||||||
|
Male |
8 (5.5) |
137 (94.5) |
1 |
1 |
||
|
Female |
18 (17.6) |
84 (82.4) |
3.67 (1.53-8.81) |
0.03 |
1.54 (0.35-6.83) |
0.06 |
|
Occupation |
||||||
|
Farmer |
2 (4.9) |
39 (95.1) |
1 |
1 |
||
|
Merchant |
8 (9.8) |
74 (90.2) |
2.11 (0.43-10.41) |
1.33 (0.21-8.42) |
||
|
House wife |
14 (24.6) |
43 (75.4) |
6.35 (1.36-29.72) |
0.04 |
3.59 (0.38-34.12) |
0.1 |
|
Civil servant |
1 (2.1) |
46 (97.9) |
0.42 (0.04-4.86) |
0.31 (0.02-5.03) |
||
|
Others* |
1 (5.0) |
19 (95.0) |
1.03 (0.09-12.04) |
0.42 (0.03-6.59) |
||
|
Symptom |
||||||
|
Symptomatic |
10 (20.4) |
39 (79.6) |
2.92 (1.23-6.91) |
0.02 |
2.74 (0.92-8.13) |
0.07 |
|
Asymptomatic |
16 (8.1) |
182 (91.9) |
1 |
1 |
||
|
Duration of DM |
||||||
|
<5year |
13 (8.7) |
137 (91.3) |
1 |
1 |
||
|
>=5year |
13 (13.4) |
84 (86.6) |
1.63 (0.72-3.69) |
0.07 |
1.30 (0.48-3.55) |
0.15 |
|
Medication for DM |
||||||
|
Tablet |
13 (8.7) |
137 (91.3) |
1 |
1 |
||
|
Insulin |
12 (16.2) |
62 (83.8) |
2.04 (0.88-4.73) |
0.23 |
1.93 (0.66-5.66) |
0.12 |
|
Both |
1 (4.3) |
22 (95.7) |
0.48 (0.60-3.85) |
0.50 (0.05-4.61) |
||
|
History of previous UTI |
||||||
|
Yes |
4 (22.2) |
14 (77.8) |
2.69 (0.81-8.88) |
>0.05 |
1.58 (0.34-7.46) |
>0.05 |
|
No |
22 (9.6) |
207 (90.4) |
1 |
|||
|
BMI (kg/m2) |
||||||
|
<25 |
11 (7.5) |
136 (92.5) |
1 |
1 |
||
|
25-29.9 |
8 (9.9) |
73 (90.1) |
1.36 (0.52-3.52) |
2.21 (0.70-6.97) |
||
|
>=30 |
7 (36.8) |
12 (63.2) |
7.21 (2.36-22.03) |
<0.01 |
14.44 (3.55-58.77) |
<0.01 |
Table 3 Statistical analysis of independent variables with respect to their contribution to significant bacteriuria from March- May, 2018 at HUCSH, Hawassa, South Ethiopia
*Student, Daily labor; CI, confidence interval; BMI, body mass index
Antimicrobial susceptibility profile
Antimicrobial resistance level of Gram negative bacteria isolates were ranged from 5.9% to 82.4%. From Gram negative bacteria isolates, high rate of resistant (82.4%) was observed against AMP and TE. On the other hand, high rate of sensitivity (94.1%) was observed against F and NOR. All (100%) E.coli isolates were sensitive to F and NOR, and 75% of the isolates (9/12) were sensitive to CRO and CIP. E.coli isolates also showed high resistant to TE 11(91.7%) and AMP 10(83.3%). All Klebsella species isolates were sensitive to CN and all of the isolates were found to be resistant to AMP. Single Acetnobacter species isolate was sensitive to AMP, AMC, CRO, F and NOR (Table 4). Antimicrobial resistance level of Gram positive bacteria isolates were ranged from 0% to 100%. All Gram positive isolates showed resistance against SXT. On the other hand all of isolates were found to be sensitive to AMC. CONs were the predominant gram positive isolate which showed 7(100%) sensitivity to AMC and 6(85.7%) sensitive to CRO. On the other hand these bacteria were found to be resistant to SXT (100%) and TE 6(85.7%) (Table 5).
|
Bacteria |
Total |
S/R |
AMP |
AMC |
SXT |
CN |
CRO |
F |
NOR |
NAL |
TE |
CIP |
|
E.coli |
12 |
S |
2(16.7) |
7(58.3) |
4(33.3) |
7(58.3) |
9(75.0) |
12(100) |
12(100) |
3(25.0) |
1(8.3) |
9(75.0) |
|
R |
10(83.3) |
5(41.7) |
8(66.7) |
5(41.7) |
3(25.0) |
0(0) |
0(0) |
9(75.0) |
11(91.7) |
3(25.0) |
||
|
K. pneumoniae |
2 |
S |
0(0) |
1(50.0) |
2(100) |
2(100) |
1(50.0) |
1(50.0) |
2(100) |
1(50.0) |
0(0) |
2(100) |
|
R |
2(100) |
1(50.0) |
0(0) |
0(0) |
1(50.0) |
1(50.0) |
0(0) |
1(50.0) |
2(100) |
0(0) |
||
|
K. oxytoca |
2 |
S |
0(0) |
0(0) |
0(0) |
2(100) |
0(0) |
2(100) |
1(50.0) |
1(50.0) |
2(100) |
0(0) |
|
R |
2(100) |
2(100) |
2(100) |
0(0) |
2(100) |
0(0) |
1(50.0) |
1(50.0) |
0(0) |
2(100) |
||
|
Acetnobacter spp |
1 |
S |
1(100) |
1(100) |
0(0) |
0(0) |
1(100) |
1(100) |
1(100) |
0(0) |
0(0) |
0(0) |
|
R |
0(0) |
0(0) |
1(100) |
1(100) |
0(0) |
0(0) |
0(0) |
1(100) |
1(100) |
1(100) |
||
|
TOTAL |
17 |
S |
3(17.6) |
9(52.9) |
6(35.3) |
11(64.7) |
11(64.7) |
16(94.1) |
16(94.1) |
5(29.4) |
3(17.6) |
11(64.7) |
|
R |
14(82.4) |
8(47.1) |
11(64.7) |
6(35.3) |
6(35.3) |
1(5.9) |
1(5.9) |
12(70.6) |
14(82.4) |
6(35.3) |
Table 4 Antimicrobial susceptibility patterns of Gram negative bacteria isolated from diabetic patients with UTI from March- May, 2018 at HUCSH, Hawassa, South Ethiopia
S, sensitive; R, resistance; AMP, ampicillin; AMC, amoxicillin- clavulanic acid; SXT, trimethoprim-sulphamethoxazole; CN, gentamicin; CRO, ceftriaxone; F: nitrofurantoin; NOR, norfloxacine; NAL, nalidixic acid; TE, tetracycline; CIP, ciprofloxacin
|
Bacteria |
Total |
S/R |
AMP |
AMC |
SXT |
CN |
CRO |
F |
NOR |
TE |
CIP |
P |
|
CONs |
7 |
S |
2(28.6) |
7(100) |
0(0) |
4(57.1) |
6(85.7) |
4(57.1) |
4(57.1) |
1(14.3) |
4(57.1) |
3(42.9) |
|
R |
5(71.4) |
0(0) |
7(100) |
3(42.9) |
1(14.3) |
3(42.9) |
3(42.9) |
6(85.7) |
3(42.9) |
4(57.1) |
||
|
S.aureus |
2 |
S |
0(0) |
2(100) |
0(0) |
2(100) |
0(0) |
2(100) |
1(50.0) |
0(0) |
1(50.0) |
0(0) |
|
R |
2(100) |
0(0) |
2(100) |
0(0) |
2(100) |
0(0) |
1(50.0) |
2(100) |
1(50.0) |
2(100) |
||
|
TOTAL |
9 |
S |
2(22.2) |
9(100) |
0(0) |
6(66.7) |
6(66.7) |
6(66.7) |
5(55.6) |
1(11.1) |
5(55.6) |
3(33.3) |
|
R |
7(77.8) |
0(0) |
9(100) |
3(33.3) |
3(33.3) |
3(33.3) |
4(44.4) |
8(88.9) |
4(44.4) |
6(66.7) |
Table 5 Antimicrobial susceptibility patterns of Gram positive bacteria isolated from diabetic patients with UTI from March- May 2018 at HUCSH, Hawassa South Ethiopia
S, sensitive; R, resistance; AMP, ampicillin; AMC, amoxicillin- clavulanic acid; SXT, trimethoprim-sulphamethoxazole; CN, gentamicin; CRO, ceftriaxone; F, nitrofurantoin; NOR, norfloxacine; TE, tetracycline; CIP, ciprofloxacin; P, penicillin; CONs, coagulase negative staphylococcus
Multidrug resistance pattern of the isolates
Multidrug resistance (resistance to two or more different class of drugs) was observed in 19(73.1%) of bacterial isolates. Of which 11(57.9%) and 8(42.1%) were Gram negative and Gram positive bacteria, respectively. Nine (75.0%) of the E. coli isolates were MDR Table (6 & 7).
|
Antibiotics |
E.coli |
K.pneumoniae |
Total |
|
SXT,TE |
1 |
1 |
|
|
NA,TE |
1 |
1 |
|
|
AMP,TE |
2 |
2 |
|
|
AMP,AMC |
2 |
2 |
|
|
AMP,NA,TE |
1 |
1 |
|
|
AMP,SXT,TE |
1 |
1 |
|
|
AMP,SXT,TE,CN |
1 |
1 |
|
|
AMP,SXT,CRO,AMC,NA |
1 |
1 |
|
|
AMP,SXT,AMC,NA,CIP,P |
1 |
1 |
|
|
Total |
9(81.8%) |
2(18.2%) |
11(100%) |
Table 6 Multi-drug resistance pattern of Gram negative bacteria isolated from diabetic patients with UTI from March- May 2018 at HUCSH, Hawassa, South Ethiopia.
AMP, ampicillin; AMC, amoxicillin- clavulanic acid; SXT, trimethoprim-sulphamethoxazole; CN, gentamicin; CRO, ceftriaxone; F, nitrofurantoin; NOR, norfloxacine; NAL, nalidixic acid; TE, tetracycline; CIP, ciprofloxacin
|
Antibiotics |
CONs |
S.aureus |
Total |
|
CIP,NOR |
1 |
1 |
|
|
SXT,NOR |
1 |
1 |
|
|
SXT,CIP,P |
1 |
1 |
|
|
AMP,SXT,P |
1 |
1 |
|
|
AMP,SXT,TE,CIP |
1 |
1 |
|
|
AMP,SXT,TE,P |
1 |
1 |
|
|
AMP,SXT,TE,P,CRO |
1 |
1 |
|
|
AMP,SXT,TE,NOR,CIP |
1 |
1 |
|
|
Total |
6(75%) |
2(25%) |
8(100%) |
Table 7 Multi-drug resistance pattern of Gram positive bacteria isolated from diabetic patients with UTI from March- May 2018 at HUCSH, Hawassa, South Ethiopia
AMP: ampicillin; AMC: amoxicillin- clavulanic acid; SXT: trimethoprim-sulphamethoxazole; CN: gentamicin; CRO: ceftriaxone; F: nitrofurantoin; NOR: norfloxacine; TE: tetracycline; CIP: ciprofloxacin; P: penicillin; CONs: coagulase negative Staphylococcus
Urinary tract infection is the commonest bacterial infectious disease with a high rate of morbidity and financial cost. The risk of developing infection in diabetes is higher due to abnormalities in the host defense and high glucose in urine.11 In the present study the overall prevalence of significant bacteriuria in diabetic patients was 10.5%. This is similar to the findings reported previously in Addis Ababa (10.9%),6 Debre Tabor (10.9%)12 and Romania (10.7%),13 but lower than a study done in Gondar (17.8%)4 and other studies done in Sudan (19.5%),7 Nepal (21%),14 Iraq (35.3%),15 and Pakistan (51%).16 This variation in prevalence might be due to the difference in sample size, geographical location, personal hygiene, and variation in the screening test used. In our study the most frequently isolated bacterial uropathogens were E. coli 12(46.2%), CONs 7(26.9%), K. pneumoniae 2(7.7%), K. oxytoca 2(7.7%) and S. aureus 2(7.7%). The predominant bacteria isolate in our study was E. coli. This is similar with previous study finding in Ethiopia (31.7%) and other countries in Sudan (56.4%), Nigeria (46%), and Cameroon (48%).4,7,17,18 E. coli considered the most predominant uropthogen due to a number of virulence factors specific for colonization and invasion of the urinary epithelium.19,20 The second most common isolate was CONs (26.9%) which is similar with a study done in Gondar (22.0%).4 In other hand it is contradicting with a study done in Addis Ababa 28%,6 Sudan (23%), Uganda (28.6%), India (20%), Iraq (15.1%) and Nepal (21.6%).7,11,15,21,22 The higher isolation rate of CONs in this study could be change in pattern of infection in diabetic patients.11
In the present study BMI >=30kg/m2 was 14 times more likely to develop significant bacteriuria (AOR=14.44; 95%CI=3.55-58.77; p<0.01). This is in agreement with a study done in Saudi Arabia23 and Spain.24 But it disagree with study result in Iran where BMI and UTI showed no association.25 In our study age range with 40-59 years were 80% less likely to develop significant bacteriuria compared with age range of 18-39 years (AOR=0.19; 95%CI=0.05-0.65; p<0.01). A study in Gondar also showed 30.7% of bacteriuria within 20- 35 age range, but not statistically significant.4 Other studies in Sudan,7 and Saudi Arabia23 reported that no significant association between age and significant bacteriuria. The variation may be due to the difference in distribution and categories of age. From our study significant bacteriuria was high among female diabetic patients (17.6%) than male diabetic patients (5.5%). This is in agreement with many other studies done in Gondar, Ethiopia (21.2%), Debre Tabor, Ethiopia (16.3%), Uganda (16.4%), and Romania (15.3%),4,12,13,21 but contradict with a study done in Sudan which is higher prevalence in male (21.2%) than female (14%).7 This could be due to short urethra, close proximity of the urethra to the anus, sexual activity, decrease of normal vagina flora, less acidic pH of vaginal surface, lack of prostate secretion, and poor hygienic condition may access entry of bacteria in to bladder and cause infection.4,11
In our study the frequency of UTI was higher among duration of DM greater than 5 years (13.4%) compared to those duration of DM less than 5 years (8.7%), although not statistically significant (p>0.05). This is in agreement with a study done in Gondar,4 Sudan,7 Saudi Arabia23 and Iran26 and disagree with a study done in Romania where longer duration is a significant risk factor associated with UTI.27 Duration of diabetes had been described as risk factor for complicated UTI, probably because of concurrent neuropathy.23 Over all resistant patterns of gram negative bacteria isolates from DM patients with UTI were AMC 14(82.4%), TE 14(82.4), NAL 12(70.0%) and SXT 11(64.7%). However, 6(35.3%) resistant pattern was seen on CIP, CRO and CN. Moreover, antibiotic resistant pattern of gram positive bacteria among diabetic UTI patients were SXT 9(100%), TE 8(88.8%), AMP 7(77.7%) and 6(66.6%). However, 3(33.3%)-4(44.4%) resistant pattern was seen in CN, CRO, F, NOR, and CIP. This is similar with the report of different studies carried on the world.4,7,11,21,28 Multidrug resistance was observed in 73.1% of uropathogenic Bacteria. About 9(81.8%) gram negative bacteria were multidrug resistant among diabetic UTI patients. Also 6(75%) of gram positive bacteria was developed multidrug resistant.4,6,12 Reason for multidrug resistance of the isolate might be inappropriate and incorrect administration of antimicrobial agents as empirical treatment.
The overall prevalence of significant UTI in diabetic patients was 10.5%. The most frequently observed organisms were E. coli, CONs, K. pneumoniae, K. oxytoca and S. aureus. Sex, age, BMI, occupational status such as house wife was statistically significant. Gram negative bacteria isolates were the most common antibiotic resistant bacteria isolates from UTI patients.
We would like to acknowledge Jimma University and Hawassa University for their support. Moreover, we appreciate the study participant for their good willing.
No conflict of interest was declared by the authors.
This study was supported by Jimma University and Hawassa University College of Medicine and Health Sciences, The support included payment for data collectors and purchase of materials and supplies required for the study. The support did not include designing of the study, analysis, and interpretation of data, and manuscript preparation and publication.

©2020 Mohammed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.