eISSN: 2378-3176


Review Article Volume 5 Issue 3
Imperial College Renal and Transplant Centre, UK
Correspondence: Hsiu Lye Yap, Imperial College Renal and Transplant Centre, 5th Floor Commonwealth Building, Hammersmith Hospital campus, Du Cane Road, London W12 0NN, UK, Tel 07727 232 254
Received: August 19, 2017 | Published: September 19, 2017
Citation: Yap HL, Frankel AH, Tam FWK (2017) Review Article - MCP-1: A Potential Target for Diabetic Microvascular Complications? Urol Nephrol Open Access J 5(3): 00171. DOI: 10.15406/unoaj.2017.05.00171
MCP-1 is a potent chemokine with the ability to mobilize and stimulate leucocytes, especially monocytes and macrophages. It is increasingly recognized as an important player in the inflammatory process that is diabetic nephropathy. In this article, we describe its role in inducing renal injury by outlining key studies in animal models and clinical studies of diabetic nephropathy, its association with diabetic retinopathy, as well as its potential use as a prognostic biomarker and as a therapeutic target in the clinical setting.
Monocyte Chemoattractant Protein-1 (MCP-1) was first identified as a chemoattractant cytokine by Matsushima in 1989,1 using conditioned media from a human myelomonocytic cell line. DNA cloning and sequencing of MCP-1 was completed that same year.2 Due to its domain structure of 4 cysteine residues linked by disulphide bridges, MCP-1 is also known as Chemokine (C-C motif) ligand 2 (CCL2). It was observed to strongly attract and activate monocytes/macrophages. Apart from macrophages, MCP-1 also synchronises recruitment and infiltration of memory T cells3 and natural killer cells4 to sites of inflammation. These leucocytes have been found to release inflammatory mediators such as interleukins IL-1 and IL-6, and superoxide,5,6 which contribute to the inflammatory cascade. When MCP-1 binds to its receptor CCR2, this directly causes production of fibronectin and extracellular matrix deposition in human mesangial cells.7 Furthermore, CCR2 is also expressed on podocytes8 and renal tubular epithelial cells.9 Since the discovery of MCP-1, there have been a multitude of studies detailing its over expression and up-regulation in various diseases. This review paper will particularly focus on the role of MCP-1 in diabetic nephropathy and retinopathy, before concluding on therapies targeting MCP-1.
MCP-1 and hyperglycaemia
Ihm.10 first cultured human mesangial cells in high glucose media and found that these conditions directly stimulated MCP-1 expression, compared to normal glucose media. A hyperglycaemic state sets off a chain of metabolic events within the cell, leading to the creation of advanced glycation end products (AGEs) and reactive oxygen species (ROS).11 Yamagishi12 then demonstrated that AGEs increase MCP-1 and vascular endothelial growth factor (VEGF) secretion in human kidney mesangial cells, leading to glomerular hyperfiltration, which is a recognized feature of early diabetic nephropathy (Figure 1).
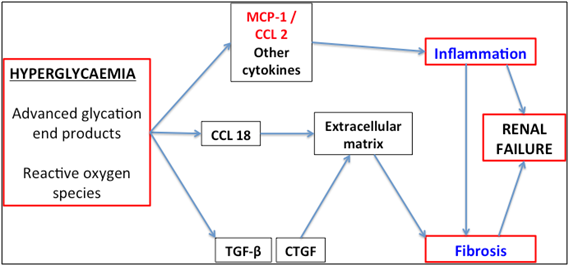
Figure 1 Pathogenesis of diabetic nephropathy. Hyperglycaemia results in the production of advanced glycation end products and reactive oxygen species, leading to cell signaling and release of MCP-1, TGF-β and CTGF. These cytokines have key roles in the inflammatory and fibrotic processes that are part of diabetic nephropathy. CCL2: CC chemokine ligand 2; TGF-β: Transforming Growth Factor Beta; CTGF: Connective Tissue Growth Factor
MCP-1 in diabetic nephropathy
The pathogenesis of diabetic nephropathy is characterized by deposition of extracellular matrix, glomerular basement membrane thickening, and glomerulosclerosis.13 In 2000, Wada14 & Banba15 found that urinary MCP-1 levels were significantly elevated in patients with diabetic nephropathy compared to healthy controls. Furthermore, urinary MCP-1 levels positively correlated with the number of interstitial macrophages and MCP-1 positive cells in kidney biopsy tissue, degree of severity of tubulointerstitial lesions14 as well as extent of albuminuria.15 It was therefore postulated that MCP-1 had a key role in inflammatory renal diseases including diabetic nephropathy, and that this was potentially mediated by macrophages.
Subsequent studies in diabetic mouse models confirmed that renal injury and progression of nephropathy is mediated by macrophages.16 MCP-1 knockout mice with streptozotocin induced diabetic nephropathy had reduced macrophage recruitment and activation in the kidneys and were protected from glomerular damage,17 thus supporting the data that suggested that MCP-1 facilitates this inflammatory process. Echoing animal studies, there is a strong relationship between the amount of glomerular and interstitial macrophage accumulation in patients with diabetic nephropathy and the rate of progression of kidney failure, as measured by serum creatinine, degree of proteinuria and interstitial fibrosis.18 Our laboratory has also shown that MCP-1 release in glomerular mesangial cells is augmented by activation of the P2X7 receptor,19 a purine ATP-gated receptor that regulates cell signaling and production of inflammatory cytokines. Increased expression of P2X7 receptors was detected in the renal biopsies of patients with diabetic nephropathy. The amount of glomerular macrophage infiltration and type IV collagen deposition were reduced in an experimental model of diabetic nephropathy in P2X7 receptor knockout mice, in comparison with that in wild type mice. Administration of a P2X7 receptor antagonist reduced the number of tubulointerstitial macrophages19 and attenuated the inflammatory response in a rat model of diabetic nephropathy.
Urinary MCP-1 was initially highlighted as a potential prognostic marker for progression of diabetic renal disease in a prospective observational study where 40 patients were followed over 6years.20 It had the most predictive merit in patients with macroalbuminuria, and levels at time zero foretold the rate of decline in eGFR better than the conventionally used urine protein/creatinine ratio. Subsequently, a larger cohort of 190 patients from the ACCORD study with persistent deterioration in eGFR over 5years were matched to 190 controls with minimal decline in eGFR over the same period.21 Patients were matched for baseline demographics, medications, glycatedhaemoglobin (HbA1c), blood pressure, eGFR- and urine albumin-creatinine ratio. The urinary MCP-1 to creatinine ratio levels at baseline and at 24months were compellingly linked to continued decline in eGFR, irrespective of degree of albuminuria.
MCP-1 in diabetic retinopathy
MCP-1 levels in vitreous humor are significantly elevated in patients with proliferative diabetic retinopathy, and correlates with extent of activity22 and clinical staging of retinopathy.23 Another report observed a strong association between MCP-1, IL-6 and IL-8 in patients with vitreoretinal diseases (including proliferative diabetic retinopathy and macular oedema), implying a common inflammatory pathway.24 Interestingly, our study describing urinary MCP-1’s prognostic role in progression of diabetic nephropathy also reported a fourfold increase in urinary MCP-1/creatinine ratio in patients who had diabetic retinopathy.20 Therefore, urinary MCP-1 levels may predict the advent of diabetic retinopathy.
MCP-1 as a therapeutic target
Currently, the mainstays of treatment for diabetic nephropathy are anti-hypertensive (including renin-angiotensin blockade) and hypoglycaemic agents. Despite these interventions, a proportion of patients can still experience progressive disease. Based on the laboratory studies, it would be interesting to investigate whether inhibiting synthesis or blocking downstream action of MCP-1 improves the outcome of patients with diabetic nephropathy. Immunomodulatory therapy shows good promise to halt or delay an almost inevitable slide to end stage renal failure. The thiazolidinedione Rosiglitazone decreased MCP-1 production and recruitment of monocytes/macrophages in human mesangial cells after mechanical stretching and exposure to high glucose media.25 Specific MCP-1 blockade has also been studied in pre-clinical and clinical settings. MCP-1 receptor antagonists used in murine models have been shown to reduce mesangial matrix deposition and macrophage driven glomerulosclerosis.26 In a clinical trial, a novel selective MCP-1 receptor antagonist CCX140-B was given in addition to standard care in a randomized, double-blind study.27 Patients were randomized to placebo, 5mg, or 10 mg of CCX140-B daily. Reduction in albuminuria was greatest in patients receiving low dose CCX140-B, indicating MCP-1 inhibition on top of ACE inhibitors or ARBs conferred further renoprotection in diabetic nephropathy. Because of the unexpected dose ranging effect of this study, further understanding of the pharmacology of MCP-1 receptor antagonistsis still needed. The effect of MCP-1 receptor antagonists in diabetic retinopathy remains to be investigated., Proof-of principle clinical studies of P2X7 receptor inhibition on renal MCP-1 production and pathogenesis of diabetic nephropathy and obesity related renal disease remain to be studied.
The pathogenesis of diabetic nephropathy is that of ongoing inflammation and fibrosis. Both experimental models and human research studies into MCP-1 have clarified its position in accelerating the inflammatory cascade, its relationship to the severity of renal lesions, and its use as a prognostic marker. More excitingly, new treatments for diabetic nephropathy targeting MCP-1 are producing results that are consistent with better outcomes compared to standard treatment, and will be welcome additions to our arsenal of weapons against this debilitating disease.
This work is supported by the Diamond Fund from Imperial College Healthcare Charity and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust. FWKT is supported by Ken and Mary Minton Chair of Renal Medicine.
Prof FWKT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, and MedImmune, and has consultancy agreements with Rigel Pharmaceuticals, Novartis and Baxter Biosciences.
None.

©2017 Yap. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.