eISSN: 2378-3176


Research Article Volume 11 Issue 2
1Bartolomé de las Casas 3765, Córdoba, Argentina
2Fundación Urológica Córdoba para la Docencia e Investigación Médica (FUCDIM), Córdoba, Argentina
Correspondence: Dr. Tristán Dellavedova, Bartolomé de las Casas 3765, Córdoba, Argentina
Received: April 29, 2023 | Published: June 6, 2023
Citation: Dellavedova T, Tristan D. Prostate specific antigen density with a cut-off point of 0.12 is the best predictor of cancer on prostate biopsies. Urol Nephrol Open Access J. 2023;11(2):37-41. DOI: 10.15406/unoaj.2023.11.00328
Introduction: Prostate cancer (PCa) is the second most frequent type of cancer in men; diagnosis is reached through prostate biopsy, an invasive procedure, so efforts are made to avoid unnecessary ones by improving them and optimizing current biomarkers. Among existing biomarkers, prostate -specific antigen (PSA) Density (PSAD), a PSA derivative, is considered a feasible biomarker for PCa
Objective: This study aimed to evaluate different PSA derivatives as well as determine whether PSAD with a cut-off point of 0.12 was more accurate than the widely used value of 0.15 to recognize patients suffering from prostate cancer.
Material and methods: Retrospective study of 391 patients aged 40 years or more, who underwent prostate biopsy at Fundacion Urologica Cordoba para la Docencia e Investigacion Medica (FUCDIM) , from November 2010 to July 2014. Sensitivity and specificity for the PSAD cut-off point of 0.12 were estimated. Diagnostic accuracy was evaluated through Receiver Operating Characteristic (ROC) curves and the Area Under the Curve. PSAD was compared to total PSA, free/total PSA index, and (free/total PSA)/PSAD.
Results: Significantly higher mean values were found in terms of total PSA, PSAD, and F/T PSA in patients with confirmed diagnoses of PCa. PSAD with a cut-off point greater than 0.12 detected a significantly higher percentage of cancer, 80%, p=0.0001, compared to the control group. Furthermore, the strength of association determined that patients with PSAD greater than 0.12 were 3 times more likely to belong to the PCa group.
Conclusion: Total PSA, PSAD, and F/T PSA in patients with cancer yielded significantly higher mean values. The best biomarker to predict prostate cancer by biopsy was PSAD, with a cut-off point of 0.12. These results indicate the need to develop further investigations in diverse geographic areas to define the best local cut-off points for PSAD. Our results demonstrate that the established cut-off point of 0.15 could be appropriate for European men but appears to be too high for American and low for Asian males.
Keywords: prostate cancer, PSA derivatives, PSA Density
Prostate cancer (PCa) is the second most frequent type of cancer in men worldwide, comprising 13.5% of the total cases (1.276.106 men), after lung cancer (14.5%, 1.368.524 patients).1 In Argentina, PCa is the most frequent type of cancer (19%) in males.1
PSA is a prostate-secreting glycoprotein that acts physiologically as serinoprotein and argininosterase; produced by prostatic epithelial cells as pro-PSA; thereupon a propeptide is removed in prostatic ducts. Its biological role consists of the liquefaction of the seminal clot.2
PSA discovery started in the late 60s, when a rapid progress in the field of immunology occurred; antigens started to be revealed in body tissues and fluids.3–5 Isolated findings were reported, and it was not until 1979, that Ming Wang and his colleagues, the main researcher from the National Prostatic Cancer Project (NPCP), purified and characterized a specific prostate antigen, demonstrating that it was present in normal, benign, and malignant tissues; their results were published in 1979.6 Papsidero et al. indicated the presence of serum PSA in patients with metastatic PCa. This was the authentic beginning of the diagnosis of PCa by a serum test.7
Nevertheless, it was not before 1991 that Catalona et al. revealed that PSA was the most accurate method for detecting PCa. It was thus the first time that PSA was used as a PCa screening test.8
In 1986, the United States (US) Food and Drug Administration (FDA) approved the use of PSA, initially for monitoring post-treatment recurrence and in 1994 for early detection of PCa.9
The introduction of PSA radically changed the history of PCa, however, after its massive use, drawbacks arose due to its lack of specificity. In this quest to improve PSA performance, investigations headed to PSA variables and derivatives.
PSA density (PSAD) is a PSA derivative obtained by the division of total PSA by prostate volume. It was first described by Benson et al. in 1989, in a publication without major significance. Benson and his colleagues continued his their research and in 1992 presented two papers in the Journal of Urology. In the first one, he they compared PSAD in patients with and without cancer. They concluded that PSAD might be useful in differentiating benign prostatic hyperplasia (BPH) from prostate cancer.10 The second paper analyzed PSAD use as an indicator of prostate biopsy; it concluded that PSAD offered significant advantages over total PSA and that its incorporation into predictive nomograms would allow more accurate cancer determination of patients with PSA at intermediate values.11
The future of PCa is unquestionably linked to genetics and molecular biology, which will allow better staging of each patient and estimate his prognosis, probability of tumor progression, and death, in order to determine the best treatment in each case.12
Despite that, until these tools become available, the use of other biomarkers, such as PSAD, is encouraged.
PSAD was chosen for this study because it is an easy-to-calculate variable, obtained from accessible data and part of the basic urological evaluation.
The cut-off point of 0.12, which fails to coincide with the widely used value of 0.15 was obtained from a previous analysis of our patients with and without cancer.13
This study aimed to evaluate PSA derivatives and to detect if PSAD with a cut-off point of 0.12 is more accurate than the internationally used value of 0.15 for the recognition of patients with PCa.
Design: a retrospective cross-sectional study of the clinical records of patients over 40 years of age treated at FUCDIM from November 2010 to July 2014. All patients underwent prostate biopsy. Indication of biopsy was based on abnormal prostate examination and/or alterations in serum PSA. Informed consent was not required according to our local ethics laws since it was a retrospective study and the confidentiality of the data was protected. Patients already diagnosed with prostate cancer, those with histological subtypes other than acinar adenocarcinoma, PSA values above 37ng/ml, and patients who did not have complete records were excluded. The final sample consisted of 391 patients.
The following data were extracted from clinical records: age, prostate volume by transrectal ultrasound, total serum PSA, free PSA, free/total ratio, PSAD, (F-T PSA)/PSAD ratio, and rectal examination. The measurement of prostate volume by transrectal ultrasound was chosen considering this determination is part of the transrectal biopsy protocol, and constitutes one of the most frequently used methods for this determination.
Summary measures were used to detail the data
Qualitative variables were expressed as absolute and relative frequency and quantitative variables as median (50th percentile) and range (maximum and minimum value). Sensitivity and specificity were estimated for cut-off points of 0.12 and 0.15. The assessment of the diagnostic accuracy of different markers was evaluated using the Receiver Operating Characteristic (ROC) curve and the area under the curve. A comparison of quantitative values between PCa patients and controls was performed with Wilcoxon nonparametric test for independent samples. For all tests, p<0.05 was set as statistical significance. The data analysis was performed with Infostat program, version 2017.
Of the total sample (n=391), 185 patients (47%) were diagnosed with PCa; their average age was 65.73 ± 0.62 years (p=0.0001) than patients without cancer (60.86 ± 0.53 years).
Table 1 shows that mean values of total PSA, PSAD, and F/T PSA were significantly higher in patients with confirmed PCa. No statistical significance of (F/T)/ PSAD index was observed.
|
Total population n=391 |
Patients with cancer (n=185) |
Patients without cancer (n=206) |
p-value |
|||||||||
|
Median |
S.E. |
Min |
Max |
P(50) |
Median |
S.E. |
Min |
Max |
P(50) |
|||
|
Marker |
PSA |
10.134 |
0.495 |
1 |
35 |
8.2 |
7.942 |
0.321 |
0.27 |
37 |
7 |
0.0051 |
|
value |
PSAD |
0.243 |
0.024 |
0.05 |
1.17 |
0.15 |
0.142 |
0.011 |
0.01 |
0.52 |
0.12 |
0.0002 |
|
F/T PSA |
0.123 |
0.007 |
0 |
0.7 |
0.11 |
0.132 |
0.005 |
0 |
0.38 |
0.13 |
0.0034 |
|
|
|
FT/PSAD |
0.75 |
0.091 |
0 |
4.6 |
0.625 |
0.958 |
0.11 |
0 |
3.4 |
0.813 |
0.1665 |
Table 1 Results of different PSA derivatives in patients with or without cancer
The accuracy of PSA markers to diagnose PCa was analyzed. Figures 1–4 shows the ROC curves and their corresponding Area Under the Curve; demonstrating that the marker that best-enabled identification of patients with PCa CaP was PSAD (66% accuracy) (Figure 1) followed by total PSA (58% accuracy) (Figure 2). Figure 3 and Figure 4 show the curves of F/T PSA index and (F/T)/PSAD, in which lower precision of these markers was observed.
Figure 5 shows that with a PSAD cut-off point of 0.12, the proportion of patients diagnosed with PCa was significantly higher (80%; p=0.0001; Chi-Square Test) than controls. In addition, the strength of the association was estimated, determining that patients were three times more likely to belong to the cancer group when presenting PSAD values greater than 0.12 (OR=3.04; CI 95% [1.84; 5.04]).
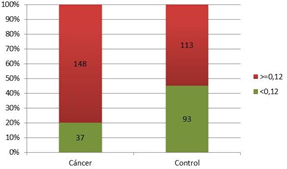
Figure 5 The proportion of patients with and without prostate cancer compared to the PSAD cut-off point of 0.12.
Figure 6 shows a significantly higher percentage of patients with PCa and PSAD values greater than 0.15 compared to controls with PSAD of 0.15 (59%; p=0.0011; Chi-Square Test). In addition, the strength of association was estimated by establishing that patients who presented PSAD greater than 0.15 (OR=2.12; CI 95% [1.35, 3.32]) were 2 times more likely to belong to the PCa group. Both the percentage of patients with CaP and the OR value for having CaP were lower than values achieved with a cut-off point of 0.12.
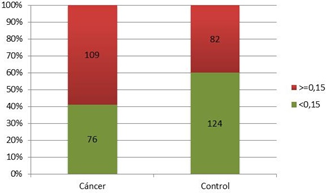
Figure 6 The proportion of patients with and without prostate cancer correlated to the PSAD cut-off point of 0.15.
Table 2 shows sensitivity and specificity values for PSAD cut-off points of 0.12 and 0.15. The 0.12 cut-off point had greater sensitivity (80%) than the 0.15 cut-off point (59%).
|
Cut-off point |
|
Sensitivity |
Specificity |
|
0.12 |
|
0.8 |
0.43 |
|
0.15 |
|
0.59 |
0.6 |
Table 2 Sensitivity and specificity values for PSAD cut-off points of 0.12 and 0.15, respectively
In this study, the marker that more accurately identified patients with prostate cancer was PSAD, yielding 66% accuracy.
After the appearance of Benson's pivotal study, multiple reports on PSAD use were published, demonstrating better results than with total PSA and other derivatives. One year later, in 1993, an Italian study analyzed PSAD in 95 patients subjected to prostate biopsy, setting for the first time the cut-off point of 0.15, which remained unchanged. Besides, this study demonstrated the usefulness of PSAD in patients with PSA values between 4-10 ng/ml, the alleged "grey zone". Based on these findings, the authors proposed that in patients with PSA of 4-10 ng/ml, PSAD could be useful, followed by biopsy when PSAD was greater than 0.15, while reevaluation after 6 months was acceptable with PSAD values lower than 0.15. This was the second contribution of this investigation, which fixed the cut-off point that remained in force until now.14
In this research, PSAD with a cut-off point of 0.12 showed that the proportion of patients diagnosed with PCa was substantially high (80%). In addition, the strength of association determined that the patients were three times more likely to be in the cancer group when they presented PSAD greater than 0.12.
In the current work, the use of PSAD with a cut-off point of 0.12 resulted in improved cancer detection, even higher than with total PSA. Due to its retrospective nature, it can be inferred that the timely application of PSAD at this cut-off point could have avoided many biopsies.
These results highlight the importance of "defying" the internationally established cut-off point and searching for the best value for each geographical area, considering ethnic and sociocultural differences.
The most commonly used cut-off point in the literature is 0.15, set by the work of Di Donna et al.14 but these reports are mainly based on studies of European populations with their own bio-demographic characteristics, that do not necessarily apply to other areas. There are no similar studies previously performed in Argentinean populations.
Our observation is supported by numerous studies on different populations from those typically analyzed.
A Chinese publication assessed the usefulness of PSAD, they evaluated different cut-off points of PSAD and the F/T PSA index. They found that PSAD was the better biomarker for PCa, and its best cut-off point was 0.13, with sensitivity and specificity of 90% and 33.7%, respectively. Thus, they concluded that PSAD was a better indicator of PCa and raised the need to redefine PSA cut-off points for the Chinese population.15
A study from Turkey, analyzed 42 men treated with radical prostatectomy and correlated total PSA, F/T PSA, and PSAD with pathology staging, finding that the cut-off point for PSAD that achieved better sensitivity and specificity in ROC curve analysis for the localized disease was 0.17.16
A Japanese work raised possible racial differences in PSA values and prostate volume, showing that the optimal cut-off point for PSAD appeared to be higher than values showed in Western literature, proposing that PSAD’s role in Asian males should be studied independently.17
A study from Kuwait, concluded that PSAD values lower than 0.32 with PSA below 10 ng/ml strongly suggested benign disease,18 challenging again the established cut-off points proposed.
Two studies performed in Indonesia support the same point of view. These authors propose that countries in the Far East with a low incidence of PCa use the Western PSAD cut-off point of 0.15 for PSA values between 4 and 10 ng/ml to indicate biopsy, but they suggest that even this value should be reanalyzed.
In one of the studies, the analysis of ROC curves indicated a PSAD cut-off point of 0.19, with sensitivity values of 100% and specificity of 79%, concluding that the PSAD value to indicate biopsy should be 0.19.19
The second Indonesian study sought the best cut-off point for PSA and PSAD to indicate biopsy and found that cut-off points of 6.95 for total PSA and 0.17 for PSAD were optimal for their population.20
A review of India revealed that PSA values according to patients’ age tended to be lower and that PSAD tended to be higher than in Western literature.21
Catalona and his co-workers, in a prospective multicenter work of almost 5000 patients, showed that a cut-off point of 0.15 increased specificity but at the cost of missing half of the tumors.22 Ten years later, his team still supported that a PSAD cut-off point of 0.10 would have acceptable sensitivity for the detection of PCa.23
Other authors have proposed different cut-off points for PSAD according to total PSA value. In 2005, Stephan et al.evaluated PSAD and F/T PSA at different ranges of total PSA. They found that PSAD differed noticeably in patients with and without PCa with different PSA and prostate volume ranges. They concluded that PSAD showed better performance than F/T PSA with PSA lower than 4 ng/ml in detecting PCa. They proposed different cut-off points for PSAD according to total PSA value; 0.05 with PSA of 2-4 ng/ml, 0.10 with a PSA of 4-10 ng/ml, and 0.19 with a PSA of 10-20 ng/ml, in order to achieve 95% sensitivity.24
A study from Brazil, in 2011, analyzed data from 1282 men referred for prostate biopsy, with PSA values of 2.610 ng/ml, searching for patterns that could reduce unnecessary biopsies, since cancer detection in their sample was 28.6%. Using a PSAD cut-off point of 0.15, they obtained a sensitivity of 74% and a specificity of 70%. When the cut-off point was lowered to 0.09, sensitivity increased to 84% and specificity to 75%. They concluded that the systematic use of PSAD as a biopsy indicator could substantially reduce the number of unnecessary biopsies.25
A paper from Taiwan analyzed PSAD, total PSA, PSA Velocity (PSAV), and F/T PSA to indicate biopsy. They found that at a 0.18 cut-off point, PSAD was superior the others. The optimal cut-off points for each variable were 6.44 ng/ml, 25%, 0.75 ng/ml/year, and 0.18 for total PSA, F/T PSA, PSAV, and PSAD, respectively. They concluded that a PSAD cut-off point of 0.18 was a significantly better predictor of PCa than the other variables studied and that it would avoid unnecessary biopsies, providing an acceptable rate of PCa detection.26
As shown, PSAD cut-off values differ from values proposed for the European populations (0.15), suggesting that lower values would be better for South and North American patients and higher values for Asian men.
This work presents, as a limitation, its retrospective nature, and the fact that the results belong to a specific population in a determined geographic location and for this reason, they cannot be directly extrapolated to other populations from different parts of the world. Another limitation would be the lack of use of multiparametric magnetic resonance, that is recognized as another tool for suspicion of cancer in the prostate.
In this study, average values of total PSA, PSAD, and F/T PSA were significantly higher in PCa patients. The greater accuracy for PCA detection was provided by PSAD (66%), followed by total PSA (58%).
PSAD with a cut-off point of 0.12 was the biomarker that worked better to detect PCa on biopsies. When compared to the 0.15 value, there was a significant difference, favoring the 0.12 cut-off point, with a sensitivity of 80% vs 59% for 0.15. The association strength showed that patients were three times more likely to present PCa with a PSAD of 0.12 versus two more times with 0.15.
In conclusion, PSAD with a cut-off point of 0.12 was the best PSA marker to predict PCa, even better than the widely used value of 0.15.
These results indicate that PSAD with a cut-off of 0.12 should be taken into consideration when assessing the need of for prostate biopsy in Argentinean patients.
The results of our study correspond to groups from specific geographic areas of Argentina. Local investigations in different parts of the world should be encouraged, searching for the validation of more appropriate values for PSA markers.
None.
Authors declare that there is no conflicts of interest to this manuscript.
None.

©2023 Dellavedova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.