eISSN: 2378-3176


Research Article Volume 8 Issue 2
Department of Internal diseases, Professor, Izhevsk State Medical Academy of the Ministry of Health of the Russian Federation, Russia
Correspondence: Yevgeniy Nikolaevich Ievlev, PhD, Assistant of the Department of Internal diseases with courses of radiation diagnostic methods and treatment, Military field therapy, Izhevsk State Medical Academy of the Ministry of Health of the Russian Federation, Address: 281 Kommunarov str., Izhevsk, 426000, Russia, Tel (3412)68-68-48
Received: March 10, 2020 | Published: April 29, 2020
Citation: Aleksandrovna KI, Ievlev YN. Features of disturbances of functions of cellular membranes in patients with chronic kidney disease, treated with programmed hemodialysis. Urol Nephrol Open Access J. 2020;8(2):57?60. DOI: 10.15406/unoaj.2020.08.00276
In recent years, there has been a steady increase in the number of patients receiving programmed hemodialysis due to the annual increase in the capacity of the dialysis centers, the progression of socially significant diseases, such as diabetes mellitus, arterial hypertension, etc. In the last decade, there has been a significant improvement in the quality of the dialysis procedure, and as a result, the number of patients with a long-term dialysis experience is growing.1–3 Stage 5 chronic kidney disease (CKD) is accompanied by a disturbance of all the functions of the body's systems and is reflected in a change of intracellular processes, including a disturbance of the structural and functional properties of cell membranes. The works from the list of references indicate that destabilization of red blood cell (RBC) membranes can be influenced by such systemic and organ factors as tissue ischemia, microbial or immune inflammation. The level of nonspecific membrane stabilizing processes reveals the nature of damage in organs and organsystems.4,8,17,19 In vivo study of the properties of cell membranes can directly and indirectly assess the regulation of all biological processes in the cells of the body.8,15 The surface electric charge of the membrane is one of the main physical characteristics of RBC, the value of which is estimated by their electrophoretic mobility.
Electrophoretic mobility of erythrocytes (EME) is the ability of RBC to move in an electric field with a speed depending on the value of their surface electric charge of the membrane.RBC must have a stable value of the surface electric charge for the adequate functioning and maintenance of homeostasis of the body. The physical and chemical state of the cell membrane (along with the composition of the environment surrounding the cell) has a crucial influence on the value of EME. In the literature there are publications indicating a decrease in the electric charge of RBC in sepsis, intestinal diseases, viral diseases, pneumonia, rheumatic diseases, as well as under stress.1–8,16,18,20 Terminal conditions, such as CKD 5D, cause changes in the density of the surface electric charge of the blood corpuscles, which are accompanied by a disturbance of the stability of the cell suspension and functional activity of cells. Thus, the electrophoretic mobility of RBC in hemodialysis patients is virtually unexplored. The emergence of new data is necessary, including, information about the impact of drug therapy on the charge of RBC membranes for the further appointment of adequate therapy.
Keywords: electrophoretic mobility of erythrocytes, chronic kidney disease, hemodialysis, hypertension, antihypertensive therapy
CKD, chronic kidney disease; RBC, red blood cell; EME, electrophoretic mobility of erythrocytes; CCB, calcium channel blockers; BB, beta-blockers; AAEO, average amplitude of erythrocyte oscillations;
The aim of the research is to identify changes in the electrophoretic mobility of RBC in patients with hypertension treated with programmed hemodialysis.
The study included 120 patients with stage 5 CKD (55%–women, 45%–men) who received program hemodialysis in Izhevsk, Udmurt Republic (BIHC of URMCH №6 of MOH of UR, BIHC of UR RCH№1of MOH of UR). The average age was 49.7±9.4 years. The procedures were performed on the devices "Fresenius " – 4008S (Germany) and B. Braun – Dialog+ (Germany) for 4-4.5 hours 3 times a week using polysulfone dialyzers. Only bicarbonate dialysis solution was used during hemodialysis. The Kt/V adequacy index for urea was higher than 1.2 and amounted to 1.43±0.09. A comparison group was created to identify the features of cell membrane disorders. The group included 60 practically healthy individuals (blood donors), comparable in age and gender. All patients were divided into 3 groups depending on the level of blood pressure (BP): normal, low and high BP. Patients with hypertension were divided into 3 groups according to its grade. Monotherapy with antihypertensive drugs included the use of slow calcium channel blockers (CCB), beta-blockers (BB) and angiotens in-converting enzyme inhibitors (ACE inhibitors).The following treatment regimens were used as combination therapy: combination of CCB and BB, CCB and ACE inhibitors, CCB and I1-imidazoline receptor agonist (IRA–Moxonidine), BB and ACE inhibitors, CCB with BB and ACE inhibitors. The groups were comparable in gender and age.
EME was measured with "CITO-Expert" complex (JSC "Axion holding company", Izhevsk, 2010) to assess membrane disorders. It provides the possibility of recording and evaluating the parameters of the movement of living cells under the influence of alternating electric field with adjusted characteristics with the light microscope "Biolam". The percentage of mobile RBC was calculated and the average amplitude of erythrocyte oscillation (AAEO) and asymmetry of oscillation were recorded. Patients underwent ABPM (apparatus MECG-DP-NS-01, 2012) for 23.2+0.6 hours. The interrelation of biochemical analyses with ABPM was evaluated. Biochemical tests were carried out in accordance with the standards of diagnostics of patients on programmed hemodialysis and included following indicators: creatinine (780.45±199.9 mmol/l), urea (29.4±6.9 mmol/l), potassium (5.33±0.47 mmol/l), sodium (137.7±2.1 mmol/l), calcium (2.52±0.5 mmol/l), phosphorus (2.1±0.4 mmol/l), alkaline phosphatase (311.7±155.2 U/l), total cholesterol(5.1±1.2 mmol/l), parathyroid hormone(899.3±728.7 pg/ml).Statistical processing of the results of the study was carried out by the generally accepted methods of variation statistics using the application programs "BioStat" (Primer of Biostatistics, Practise, Moscow, 2009, version 4.03.) and Microsoft Excel 2010 (USA). The data are presented as M±σ, where M is the mean value, σ is the standard deviation. The differences between the mean values were considered statistically significant at p<0.05. Relation analysis was performed using Spearman rank correlation coefficient (rs).
Comparing the results of the study of the main group and the comparison group, there was a significant increase in the average amplitude of erythrocyte oscillations (AAEO): the values of AAEO are 49.7+6.3 mcm and 28.4+3.2 mcm (Table 1; p<0.01) respectively. The increase can be associated with serious pathophysiological disorders in patients with CKD that affect cell membranes, which are associated with changes in the hemostatic system and mechanical damage to RBC during the passing through the dialysis filter. Significant differences in asymmetry -0.98±1.3, -1.37±0.2 (p>0.05) and excesses 5.1±6.9, 9.62±1.7 (p>0.05) respectively, weren’t observed in patients treated with programmed hemodialysis and the comparison group. The percentage of mobile RBC didn’t differ in hemodialysis patients and donors (table 1, p>0.05). There were no gender differences in AAEO: in men –50.6±6.9 mcm, in women –49±7.6 mcm (p>0.05). The differences of AAEO in patients with different duration of dialysis experience were identified: 1-6 years– 44.8±8.2 mcm, 6-10 years– 56.3±5.5 mcm, more than 11 years–50.6±2.3 mcm (р1-2<0,01, р2-3<0,01).In the first years of the dialysis therapy, there is a high mortality of patients. At this time there are gross disturbances and rearrangement of biological processes, which can justify more significant decrease in the rates of AAEO in patients with duration of dialysis therapy from 1 year to 6 years (p<0.01).
Indicator |
Hemodialysis patients |
Donors |
N=120 |
N=42 |
|
AAEO (mcm) |
48,9±6,7*** |
28,4±3,2 |
Asymmetry of oscillation |
-0,98±1,3 |
-1,37±0,2 |
The percentage of mobile RBC (%) |
98,6±15,4 |
99,2±1,2 |
Table 1 Indicators of electrophoretic mobility of RBC
Note: The reliable indicator of differences between groups – ***р<0,0001.
It was found that the etiological factor of CKD, such as polycystic kidney disease, more significantly reduced AAEO (48.6±4.5 mcm; p<0.01). This probably connected with genetically determined disorders of the structure of cell membranes, as well as the presence of chronic inflammation and severe hypertension. The value of AAEO in patients with chronic glomerulonephritis was 49.5±6.3 mcm, with diabetic nephropathy – 50.7±7.2 mcm, with secondary chronic pyelonephritis –54.7±4.1 mcm (p<0.01). Differences in AAEO were identified in patients with hypertension and normal BP: 47.8=6.2 mcm and 54.3=3.7 mcm respectively (p<0.01). Patients with hypertension had lower (negative) values of erythrocyte oscillation asymmetry than patients with normal BP (-1.3±1.2 and -0.35±0.8 respectively; p<0.01). No statistically significant differences in the percentage of mobile RBC were obtained in patients with hypertension, with high and low BP, (Table 2). In individuals with 1 grade hypertension, the AAEO was 47.5±1.4 mcm, with 2 grade hypertension –47.6±2.8 mcm, with 3 grade hypertension –52.7±3.2 mcm (p<0.01; Table 3). Differences in rate of asymmetry of oscillation of RBC were found. In patients with grade 1 hypertension, the percentage of mobile RBC was significantly higher than in those with grade 3 hypertension (98.3±0.7% and 96.0±0.9%, respectively; p<0.05).
Indicator |
Normal BP |
Hypertension |
Hypotension |
N=15 |
N=85 |
N=20 |
|
47,8±6,2** |
46,1±7,7 |
||
Asymmetry of oscillation |
-0,35±0,8 |
-1,3±1,2* |
-1±1,5 |
The percentage of mobile RBC (%) |
98,8±1,1 |
98,2±2,9 |
98,8±1,4 |
Table 2 Comparative characteristics of electrophoretic mobility of RBC in groups with different BP levels
Note: The reliable indicator of differences between groups – **р<0,01; *р<0,05 –in comparison with group of patients with normal BP
Indicator |
1 Grade |
2 Grade |
3 Grade |
N=44 |
N=30 |
N=11 |
|
AAEO (mcm) |
47,5±1,4** |
47,6±2,8 |
52,7±3,2 |
Asymmetry of oscillation |
-1,31±0,32 |
-2,44±0,25 |
-1,49±0,52 |
The percentage of mobile RBC (%) |
98,3±0,7* |
98,5±0,5 |
96,0±0,9 |
Table 3 Comparative characteristics of electrophoretic mobility of RBC in groups with different grades of hypertension
Note: The reliable indicator of differences between 1st and 3rdgroups *p<0,05; **p<0,01.
The decrease in AAEO in patients with hypertension can be explained by a disturbance of the structure and function of cell membranes, which are that facilitated ion transfer through the cell membrane proceeds with greater speed in the presence of hypertension.6,14 In the study of hypertension in patients on programmed hemodialysis Boero et al.14 measured RBC Na/K ATPase activity and noted lower pump activity in patients with hypertension as opposed to patients with normal BP.2 In further studies, data on the inhibition of Na/K ATPase are presented, due to which there is an increase in intracellular sodium and cytosolic calcium, leading to increase in level of basal vascular tone and vascular response to vasoconstrictors.6,2,13 During ABPM, high correlations of AAEO with the nocturnal BP decrease(SBPrs=0.73, p<0.001; DBPrs=0.63, p<0.001) and heart rate (rs=-0.64, p<0.001) were proven. Nocturnal BP decrease is an indicator not only determining the type of circadian rhythm of BP, but also significantly affecting the progression of left ventricular hypertrophy, the risk of cardiovascular complications and, eventually, the survival rate of patients with hypertension. Thus, the disturbance of cell membranes can determine the prognosis of the disease, which in turn determines the strategy of antihypertensive and organ-protective therapy. Therefore, a comparison of membrane disorders in different antihypertensive therapy was carried out.
In patients receiving CCB therapy, the level of AAEO was 51.5±5.4 mcm; ACE inhibitors– 44.6±5.8 mcm; BB – 46.6±1.1 mcm (p1-3<0.05; p1-2>0.05, p2-3<0.05). The asymmetry of erythrocyte oscillation in patients receiving ACE inhibitor therapy was -2.4±0.3, CCB –-0.6±1.4; BB – 0.2±0.3 (p1-3<0.05; p1-2<0.05, p2-3>0.05) (Figure 1). Several studies have shown that CCB can protect the cell from damage of plasma membranes (ischemic, physical or chemical), leading to apoptotic cell death or necrosis in conditions of prolonged increasingof intracellular concentration of Ca2+.10–12 This conclusion confirms the data obtained by us about the positive effect of CCB monotherapy on the electric charge of the RBC membrane (p<0.05). Also, in the absence of CCB in combined antihypertensive therapy, the lowest rates of AAEO were observed (combination of BB and ACE inhibitors; 42.3=6.3 mcm, p<0.05).
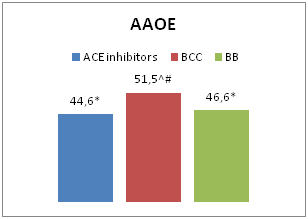
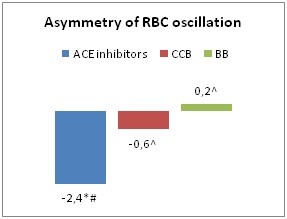
Figure 1 Characteristics of EME in patients receiving monotherapy.
Notes: In comparison with patients received CCB therapy:*- р<0,05; ACE inhibitors:^- р<0,05; BB: #- р<0,05.
The triple combination (CCB, BB, IRA) more effectively stabilized the charge of cell membranes, which was confirmed by a higher level of AAEO in patients (56.3=6.9 mcm; p<0.01).The obtained results indicate the need for the use of triple combination in low-dose regimens in patients with hypertension receiving programmed hemodialysis. High rates of AAEO were also revealed in patients taking CCB with IRA, CCB with ACE inhibitors, CCB with BB (53.7±3.7, 51.6±5.8, 48.0±3.4 mcm respectively, Figure 2). Thus, among the double combinations, the combination of CCB and IRA is most effective in stabilizing the charge of cell membranes. A number of studies have shown the role of autonomic homeostasis disorders in the pathogenesis of hypertension in patients with CKD, in this regard, the use of selective IRA is necessary.3,9 Our data show that IRA can positively affect the properties of cell membranes of the body, which can open up new priorities in their appointment.
Indicators of electrophoretic mobility of erythrocytes in patients treated with programmed hemodialysis, have their own characteristics, in the form of a significant increase in the average amplitude of erythrocyte oscillations (48.9±6.7 mcm), compared with the group of practically healthy individuals (28.4±3.2 mcm).The level of AAEO depends on the duration of dialysis therapy (the level is lower, if the duration is from 1 year to 6 years), etiological factor (the level is lower in case of polycystic kidney disease), the presence and grade of hypertension. The value of AAEO is highly correlated with the level of nocturnal BP decrease. High EME values were determined in monotherapy with slow calcium channel blockers and triple antihypertensive therapy (BB, CCB, IRA).
None.
The author declares there is no conflict of interest.
None.

©2020 Aleksandrovna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.