eISSN: 2378-3176


Case Report Volume 7 Issue 3
1Dr Soliman Fakeeh Hospital, KSA
2Associate professor, Fakeeh College medical sciences, KSA
3Urology & Nephrology Center, Mansoura University, Egypt
Correspondence: Ahmed AKL, Associate Professor, Fakeeh College Medical Sciences, Jeddah, KSA Consultant Nephrology & Transplantation, DR. Soliman Fakeeh Hospital, Jeddah, KSA, Consultant Nephrology & Transplantation, Urology & Nephrology Center, Mansoura University, Egypt
Received: May 06, 2019 | Published: July 10, 2019
Citation: Ahmed AKL, Aldabbagh Y. Does gymnasium harbor an endemic cause of end stage kidney disease? a case report & review of literature. Urol Nephrol Open Access J. 2019;7(3):55-57. DOI: 10.15406/unoaj.2019.07.00244
Gymnasium is an English word describing a place for physical training and simply referred to as Gym. Nowadays, in Gym physical training is associated with consumption of high protein and non-protein supplements aiming to have effective muscle and bone building in short time. Those supplements accompanied with strenuous exercise and dehydration results in high incidence of acute kidney injury. We report a 26-year-old male, casual bodybuilder, giving positive history of anabolic steroids intake, creatine supplements consumption, high protein diet, and using energy drinks for rehydration, Accidentally discovered to have end stage kidney disease, His Urine analysis was unremarkable, His Renal ultrasound showed grade III hyper-echogenicity and his Renal biopsy revealed severe renal cortical damage, glomerulosclerosis percentage was 100%, IFTA percentage was 90%, non-specific C3 staining of one glomerulus, Electron microscopy was not conclusive as all glomeruli were sclerotic in the specimen. In conclusion, Gym nephropathy is multifactorial and can be prevented by spreading the knowledge of healthy eating, good hydration during training and unleash the dangers of high protein and non-protein supplements for bodybuilding.
Keywords: creatine, acute intestinal nephritis, acute renal failure, chronic renal failure, high protein diet, branched chain AA, energy drinks
Gymnasium is the Latin word for old Greek word gymnasion which means a place where young men practice physical & intellectual activities. It is a place of intellectual activities which can be found in European languages. In contrary, Gymnasium in English language is defined as being a place for physical activities only and shortened to the form “Gym”. Nowadays, in Gym, athelets beside the practice of physical activity, they frequently consume non hormonal and hormonal supplements in order to acquire a muscle bulk and strength in a short time.1 The main non-hormonal supplements include proteins, creatine and vitamins, and the hormonal supplements are the anabolic steroids.2 Recently reported in literature that 10 body builders suffered chronic kidney disease due to focal segmental glomerulosclerosis (FSGS) after consuming large amounts of anabolic steroids with high protein daily intake of 300–550 g/day.3 The high-protein intake carries a lot of concern because it increases glomerular filtration rates and proved experimentally to be associated with glomerular hyper filtration ending with FSGS.2 Furthermore, pure creatine powder is marketed as a muscle building supplement. It is safe & effectively contributing to exercise tolerance and improve muscle strength.4,5 Creatine is widely used by casual and professional athletes.6 The reported adverse events is usually because of the exercise-induced rhabdomyolysis and dehydration leading to acute kidney injury.7 However, many cases report un-linking creatine-associated acute kidney injury to rhabdomyolysis.8–11 A one report describing a body builder suffered from acute tubular necrosis after taking a high dose up to 20 g/day for a week.9 Another report, describing acute intestinal nephritis in two body builders.10,11
Sports dieticians sometimes recommend higher doses of vitamin D over the recommended daily allowances (RDA) to strength bone building.12,13 A sustained ingestion of over 50 000 IU a day can cause hypercalcemia with the potential for metastatic renal calcification.12 Vitamin D toxicity, usually seen in hospitalized infants as a result of over dose, but considered minimal and rarely seen in adults in normal situations.13,14 Anabolic androgen steroids (AAS) proved to be directly toxic to podocytes through stimulation of podocyte androgen receptors leading to podocyte loss and segmental sclerosis.15 In addition, AAS side effects includes an increase in the risk to have coronary attack, acute myocardial infarction, cholestatic jaundice, transaminitis, rhabdomyolysis plus severe mood and psychotic disorders.16 Herein, we report our case to alert nephrologists and general physicians to bodybuilders behaviors and their trainers that may place young men at risk for new era of kidney injury we named gym nephropathy.
A 26-year-old male, active bodybuilder, accidently discovered low hemoglobin level when he was donating blood, his HB was 8 g/dl and serum creatinine 12 mg/dl, BUN 118 mg/dl and metabolic acidosis PH 7.2, and serum bicarbonate was 14 mmol/l. No previous medical history, his sister known to have systemic lupus (SLE). Renal us done and revealed average size kidneys 8x5 cm with grade III hyper-echogenicity, urine analysis was unremarkable, Patient gave positive history of anabolic steroids intake, creatine supplements consumption, high protein diet, and he was using energy drinks for rehydration. Immunological work up including anti-neutrophilic antigen (ANA), C3, C4, ESR, CRP all came normal. Renal biopsy included up to 19 glomeruli, all of them globally sclerosed. Tubular atrophy and interstitial scarring affect about 90% of the cortical area. Surviving proximal tubular epithelium shows cytoplasmic protein reabsorption droplets and hypertrophic changes focally. Five small arteries are included that show fibro-intimal thickening. Thrombotic microangiopathy is not seen Figure 1(A) & Figure (B). Immunofluorescence Microscopy showed sections stained for Albumin, Fibrinogen, C1q, C3, IgG, IgM, IgA, Kappa light chain & Lambda light chain. There is positivity for C3 (3+) in sclerosed glomeruli. Traces of mesangial IgM are seen in sclerosed glomeruli. Other immune-stains are negative except for IgA positivity of hyaline casts and positivity for IgM, IgA, Kappa and lambda chains in atrophic tubular basement membranes Figure 1(C). Electron microscopy was not conclusive as all glomeruli were sclerotic in the specimen [Biopsy pathology processing and analysis was done at Express Med laboratories, Kingdom of Bahrain]. He received normal strength saline (77 meq sodium bicarbonate in one-liter ½ NSS) at rate of 2 ml/kg/hour, urine output was satisfactory 1300-1600 ml/day. After two days of IV fluids serum creatinine showed partial improvement from 12 to 10 mg/dl and BUN 118 to 98 mg/dl. In view of the partial improvement of kidney function and possibility of patchy kidney sclerosis, we started the patient on low dose oral steroid dose with monitoring of kidney function. After two weeks, no response to steroids was observed, patient was declared to have end stage renal disease (ESRD) and prepared to initiate regular hemodialysis and kidney transplantation option was discussed with the patient.
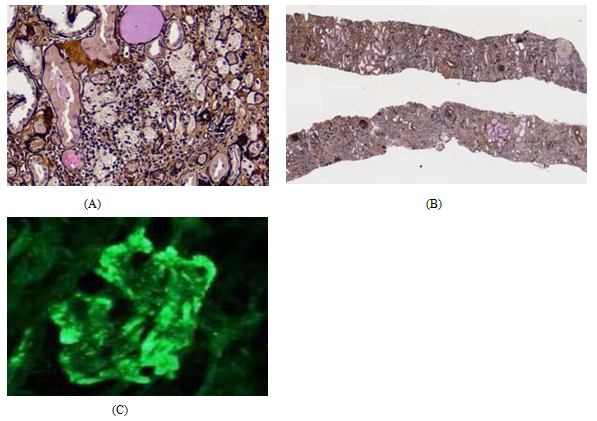
Figure 1 Histopathology of core needle renal biopsy.
Figure 1: shows (A, B) Light Microscopy: severe chronic renal parenchymatous damage, glomerulosclerosis 100%; IFTA 90%. (C) Immunofluorescent microscopy: There is positivity for C3 (3+) in sclerosed glomeruli. Traces of mesangial IgM are seen in sclerosed glomeruli. Other immunostains are negative except for IgA positivity of hyaline casts and positivity for IgM, IgA, Kappa and lambda chains in atrophic tubular basement membranes.
Creatine supplement increases creatine storage and promotes a rapid regeneration of adenosine triphosphate in-between extensive exercises. Nowadays, its use is increasingly observed among Gym athletes. Studies have shown that protein supplement, when it is used in doses more than recommended, it could be associated with acute tubular necrosis or acute intestinal nephritis and tend to resolve with stopping of protein supplements.3–4 Our patient was not following the dose recommended by his coach as he was taking triple the prescribed creatine and protein supplements. A significant chronicity was found in renal biopsies of patients on maintenance protein supplements. However, it is difficult to rule out a pre-existing chronic kidney disease that may be associated with supplements use.5 Our patient family history was positive for systemic lupus nephritis but all the immunological screening were negative. Many reports correlating the incidence of acute kidney injuries in Gym trainees to consumption of anabolic steroids with moderately high amounts of commercial nutritional supplements containing protein & creatine in doses exceeding the allowed recommended dose on a continuous basis. Published renal biopsies demonstrated acute tubular injury, and when the anabolic steroids & protein supplement utilization stopped early, kidney function improved within 4 weeks.
This raised the concern of the association between high protein supplement use and ESRD. A number of Gym trainees suffered hypercalcemia, ESRD with nephrocalcinosis while consuming vitamin D with estimated doses of >10 million IU annually. This finding raised the possibility of vitamin D toxicity induced nephrocalcinosis encountered in four Brazilian Gym trainees.17–19 The nephrocalcinosis was described to be due to the silicon-like effect of oily carrying medium that was added to vit D injections that was used to increase specific muscle groups bulk.17–19 Vitamin D toxicity is the milk- or calcium alkali syndrome.20–22 An excessive consumption of milk or anti-acid calcium carbonate compounds leads to this syndrome. Nowadays, the calcium-alkali syndrome is seen in post-menopausal women receiving supplemental calcium & vitamin D for osteoporosis. Among our patient, milk & calcium consumption could not be considered greatly excessive. Acute phosphate nephropathy with intratubular calcium phosphate deposition was reported in subjects receiving oral sodium phosphates used for bowel preparation before colonoscopy.23 Protein powder contains little inorganic phosphate with three daily supplement servings providing <0.5 g.24 Creatine is sold mainly in the form of a monohydrate also with little phosphorus. Daily 2–3 Liters of milk consumption will add 1.6–2.4 g to a normal dietary phosphorus intake of 1.0–1.5 g.24 This is well below the 11 g oral intake used in bowel preparations.23
For most Gym trainees, sports dieticians recommend a daily protein intake of 1.4–1.7 g/kg/day.2,25 There is substantial experimental and clinical data supporting the safety of creatine supplementation when it is used in the recommended amounts, but there is concern that excess dietary protein and creatine that is not accompanied by increased fluid intake may lead to a relative hypovolemia.5–8 In reported cases of acute kidney injury in creatine users; one demonstrated acute tubular necrosis, but two were classified as acute interstitial nephritis suggesting idiosyncratic allergic reactions.9–11 We must emphasize that a specific offending agent cannot be identified in our case, and it may be the combination of excess creatine, protein with anabolic steroids injections that compounds risk not incurred with the individual substances. In conclusion: we encourage youth to practice a healthy lifestyle and sports. We are presenting this case to focus on a serious and hidden cause of end stage renal disease emerging in young population due to consumption of high protein supplements, anabolic steroids and creatine during practicing sports in gym. This danger can be prevented through acknowledgement of a public alert program.
The present study was performed in accordance with the ethical standards of the institutional research committee and with the Helsinki declaration.
Written consent was obtained from the patient discussed and documentation is available for review upon request.
None
Authors declare No conflict of interest.

©2019 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.