eISSN: 2378-3176


Research Article Volume 10 Issue 1
1Faculty of Medicine, University of Goma, Democratic Republic of the Congo
2Clinique Internationale de Mdecine Avance au Kivu, Democratic Republic of the Congo
3Institut Supérieur des Techniques Médicales de Lubumbashi, Democratic Republic of the Congo
4Department of Nephrology, Faculty of Medicine, University of Kinshasa, Democratic Republic of the Congo
5Faculty of Medicine, Université Libre des Pays des Grands Lacs, Democratic Republic of the Congo
6Faculty of Medicine, Official University of Bukavu, Democratic Republic of the Congo
7Faculty of Medicine, University of Lubumbashi, Democratic Republic of the Congo
8Faculty of Medicine, University of Kisangani, Democratic Republic of the Congo
Correspondence: Olivier Mukuku, Institut Supérieur des Techniques Médicales de Lubumbashi, Lubumbashi, Democratic Republic of the Congo
Received: June 30, 2022 | Published: July 13, 2022
Citation: Kahindo CK, Mukuku O, Mokoli VM, et al. Diagnostic value of serum creatinine versus urinary neutrophil gelatinase-associated lipocalin (uNGAL) in acute kidney injury in resource-limited settings. Urol Nephrol Open Access J. 2022;10(1):20-23. DOI: 10.15406/unoaj.2022.10.00318
Objective: To determine the diagnostic accuracy and optimal threshold for the difference between creatinine at 48 hours and creatinine at admission in hospital versus gold standard, urinary neutrophil gelatinase-associated lipocalin (uNGAL) in patients with renal impairment.
Methods: A prospective observational study was conducted from February to May 2022, with patients hospitalized in a tertiary care referral hospital. To compare the two diagnostic tests, a urine sample for the uNGAL assay using the Finecare™ NGAL rapid quantitative test, and for creatinine, a blood sample taken at admission and another after 48 hours. Only patients who had both tests were included in the study.
Results: A total of 167 patients were included. The two tests were highly correlated (r = 0.33, p<0.001). The ROC area analysis (AUC = 0.8298) demonstrated the accuracy of creatinine in the diagnosis of acute kidney injury (AKI). The optimal threshold using the Liu method was 0.3 mg/dl (sensitivity = 72.9%, specificity = 91.8%).
Conclusion: The results suggest that the difference between creatinine at 48 hours and creatinine at intake should continue to be used when diagnosing AKI. In addition, it is a simple test, easy to perform and standardize, and inexpensive compared to the uNGAL.
Keywords: diagnostic test, creatinine, uNGAL, acute kidney injury
AKI, acute kidney injury; AUC, area under ROC curve; CKD, Chronic Kidney Disease; DRC, Democratic Republic of the Congo; IQR, interquartile range; LR, negative likelihood ratio; LR, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; SCr, serum creatinine; Se, sensitivity; Sp, specificity; uNGAL, urinary neutrophil gelatinase-associated lipocalin
Acute kidney injury (AKI) is the source of a significant medical-surgical mortality that develops in different clinical settings, and is associated with a significant increase in health care costs as well is linked to both higher hospital costs and longer residence times.1-3 Measuring urinary neutrophil gelatinase-associated lipocalin (uNGAL) was found to be an early, non-invasive biomarker of AKI due to various etiologies. uNGAL represents a promising structural biomarker in the early detection of AKI clearly defined according to the criteria of KDIGO (Kidney Disease: Improving Global Outcomes) classifications.4,5 It has been shown to be more sensitive and specific for the detection of AKI in emergencies and for the prediction of an overall clinical prognosis of death and dialysis after hospitalization.1,6,7 uNGAL levels can then be used to establish algorithms for the management of AKI patients earlier than serum creatinine (sCr) alone, and the use of uNGAL for diagnosis and management of AKI can potentially lead to improved patient outcomes.8,9 However, in our context of resource-constrained countries, sCr remains a test that is easier to use and more accessible than the difficult-to-find uNGAL on the market and remains expensive despite its better performance in early detection of AKI. This study raises the question of the ability of the sCr to screen for AKI versus uNGAL and whether this difference in sCr is effective in our population, although it is recommended elsewhere.10
The present study aims to determine the level of sCr performance compared to uNGAL in Goma city, in the Democratic Republic of the Congo (DRC).
This study has been approved by the Medical Ethics Board of the University of Goma (Approval Number: UNIGOM/CEM/002/2021) and informed consent was obtained prior to sampling.
The present study was a prospective monocentric observational study conducted from February to May 2022 at Virunga General Reference Hospital in Goma (DRC) to assess the performance of the difference in values of sCr (the difference between sCr at 48 hours [sCr2] and sCr at admission in hospital [sCr1]) against uNGAL. We recruited patients who were 18 years of age and older who consulted either in intensive care unit, Internal Medicine, or Surgery departments during this study period. We collected an initial urine and blood sample from patients upon admission and a prospective analysis of sCr after 48 hours of hospital admission. We had taken two values of sCr (the difference between sCr at 48 hours [sCr2] and sCr at admission in hospital [sCr1]) and evaluated its variation according to KDIGO 2012.11 and finally allowed us to determine the presence of AKI or not.
We excluded from this study all patients under 18 years of age and those undergoing dialysis or admitted for obstetric-gynecologic causes. The diagnosis of AKI was defined according to the 2012 KDIGO criteria.11 medical history, demographic characteristics, and the diagnosis as a reason for admission recorded in medical records.
Laboratory measurements
We centrifuged urine samples at 12,000 rpm for 10 minutes. Urine samples collected (morning before meal) for the uNGAL assay using the Finecare™ NGAL rapid quantitative test (Finecare Roche Diagnostics, Mannheim, Germany) using fluorescence immunoassay by a Finecare™ FIA Meter Plus automatic analyser for the quantitative determination of neutrophil gelatinase-associated lipocalin (NGAL) concentration in human urine. sCr was measured using a sCr assay kit (BioAssay Systems, Hayward, California) and was done in the central laboratory of the Clinique Internationale de Médecine Avancé in Kivu (CIMAK) using the colorimetric jaffé method for sCr with the RAYTO-brand automaton.
Statistical analysis
Statistical analysis was carried out with the statistical software STATA version 16.0.0 (StataCorp LP, StataCorp, Station College, TX, USA).
The correlation between the two tests was first established thanks to the results of the uNGAL and the difference in values of sCr obtained for each patient. With the use of the uNGAL results as the gold standard, sensitivity (Se), specificity (Sp), positive (PPV) and negative (NPV) predictive values were determined as well as the positive (LR+) and negative (LR-) likelihood ratios of the difference in values of sCr at several cut-off values for the prediction of significant sCr (≥0.3 g/dl). The diagnostic performance of the difference in values of sCr was evaluated with receiver operating characteristic (ROC) curves. In order to determine an optimal difference in sCr values as the cut-off for the diagnosis of AKI, the Liu method.12 which maximizes sensitivity and specificity, was used. A p-value less than 0.05 was considered significant.
Characteristics of the study population are presented in Table 1. The median age of the patients was 42.0 years (interquartile range [IQR]: 30.0 – 57.0), the male sex was 53.9%. Among 167 patients, 23.5% were in intensive care unit, 55.4% were in internal medicine service, and 21.1% were in surgery service. A total of 21 (15.0%) patients had diabetes mellitus, 35 (21.0%) had hypertension, and 5 (3.0%) had chronic kidney disease. The median hemoglobin level was 11.4 g/dl (IQR: 9.9 - 13.2) and median blood glucose of 106 mg/dl (IQR: 82.8 – 154.2).
Variable |
N=167 |
Mean age (years) |
42.0 (30.0 – 57.0) |
Male |
90 (53.9) |
Unit |
|
Intensive care |
39 (23.5) |
Internal Medicine |
92 (55.4) |
Surgery |
35 (21.1) |
Diabetes mellitus |
21 (15.0) |
High blood pression |
35 (21.0) |
Chronic kidney disease |
5 (3.0) |
Haemoglobin (g/dl) |
11.4 (9.9 – 13.2) |
Blood glucose (mg/dl) |
106 (82.8 – 154.2) |
Table 1 Characteristics of the study population
The correlation between the 24-hour sCr and intake sCr difference values (Figure 1) and uNGAL values is illustrated in Figure 2. There was a significant relationship between the two tests, with a correlation rate of r = 0.33 (p < 0.001). The area under the ROC curve (AUC) (Figure 3) was 0.8298.
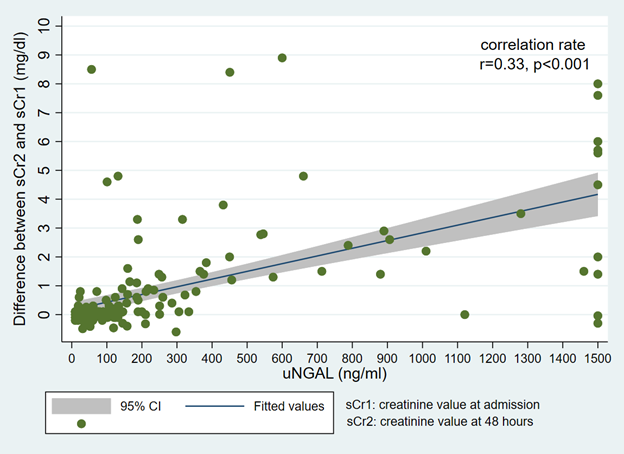
Figure 2 Correlation between the difference between 48-hour creatinine - admission creatinine and uNGAL values.
Table 2 indicates that the optimal threshold for the difference between sCr at 24 hours and sCr at intake was found to be 0.3 mg/dl (sensitivity = 72.9%, specificity = 91.8%), based on ROC analysis and using the Liu method. At this threshold, 62 patients (37.1%) had a difference of ≥0.3 mg/dl defining an AKI. Of these, 52 (83.9%) were true positives (uNGAL >131.7 ng/ml), while 10 (16.1%) were false negatives. The PPV was 83.9% and the NPV was 82.9%. The LR+ was 8.8, which means that with this threshold, it is very likely that the AKI is positive rather than negative. At a threshold of 1.5 mg/dl, 30 patients (18.0%) were positive for AKI, of whom 28 (93.3%) were true positives and two (6.7%) were false negatives. The PPV was 93.3% and the NPV was 69.3%. The LR+ is 19.4, which is higher than the previous threshold.
Cut-off point (mg/dl) |
Sensitivity (%) |
Specificity (%) |
LR+ |
LR- |
PPV (%) |
NPV (%) |
0.3 |
72.9 |
91.8 |
8.8 |
0.3 |
83.9 |
82.9 |
0.8 |
60.0 |
95.9 |
14.6 |
0.4 |
91.3 |
76.9 |
1.5 |
40.0 |
97.9 |
19.4 |
0.6 |
93.3 |
69.3 |
Table 2 Sensitivity, specificity, LR+, LR-, VPP and VPN for different thresholds
To our knowledge, this is the first study in the DRC using a urinary biomarker in a large cohort of heterogeneous patients to predict early AKI. The primary objective of this study was to assess the diagnostic accuracy of the sCr compared to the uNGAL in screening for AKI. In addition, an attempt was made to determine an optimized threshold to confirm the diagnosis. The results of this study suggest that sCr could be a good diagnostic test for early AKI in our limited-resource hospital settings, as a high correlation rate (r=0.33, p<0.001) was found with uNGAL and it had an AUC of 0.8298.
We found that the two-step sCr was able to detect impaired renal function and showed excellent performance (AUC = 0.8298) compared to the uNGAL at the beginning of hospitalization. However, some authors believe that sCr is not a very sensitive and specific marker for assessing renal function in AKI, as it is influenced by many renal and non-renal factors independent of renal function.13 This is because the sCr is an inadequate marker for AKI.14 First, substantial losses in glomerular filtration rate (GFR) may occur before an increase in the sCr is measured. Secondly, sCr does not give an accurate picture of renal function until an equilibrium point has been reached, which may take several days.13,15
This study has shown that the sCr is effective in screening for early AKI with accuracy close to uNGAL. Knowing that human NGAL, which is part of the lipocalin superfamily, was originally described as a 25 kD protein covalently bound to gelatinase in neutrophils. The expression of NGAL is induced in the injured epitheliums, especially the lung, the colon and especially the kidney.14-16 Emerging experimental and clinical evidence indicates that, in the early stages of AKI of various etiologies, NGAL accumulates in two distinct pools, namely a renal pool and a systemic pool. Studies on gene expression in AKI clearly demonstrated rapid and massive regulation of NGAL’s messenger RNA (mRNA) in the thick ascending branch of Henlé’s loop and the collecting channels, with the resulting synthesis of NGAL protein in the distal nephron (renal pool) and secretion in the urine where it constitutes the major fraction of urinary NGAL.16,17 In addition, AKI results in increased expression of NGAL’s mRNA in distant organs, including the liver and lungs, and the overexpressed NGAL protein is most likely released into the circulation and constitutes the systemic pool.14-17
The present study indicates that monitoring level of sCr can provide early warning to clinical care providers. The levels of sCr measured at the onset of hospitalization were effective in the diagnosis of AKI. The sCr could combine the accuracy of a diagnostic test with the benefits of a screening test. As a reminder, a screening test is intended to be simple and acceptable to both patients and medical personnel, with a positive threshold value generally chosen towards a high sensitivity in order to prevent the rate of false negatives.18,19 The purpose of this screening test is not to miss a possible onset of disease. For example, a positive result may lead to the suspicion of a disease that will then have to be confirmed by a more specific diagnostic test. Screening must be cheap and its benefits justify its cost so that a large part of the population can benefit in order to identify a small number of potential patients.18-20 A quantitative diagnostic test identifies a threshold for establishing the presence or absence of a pathology with the best specificity, without compromising patient safety. In this case, the goal was not to eliminate early AKI patients.19-20
Other studies have determined that uNGAL can be used but only as an exclusion test or to assess the extent of impaired renal function, especially in severe AKI, especially since the high level of uNGAL appears to be predictive of an adverse outcome of AKI.21 Nevertheless, in our context of resource-limited countries, sCr appears to be an alternative; the excellent value of AUC (0.8298) suggests that sCr could be an excellent diagnostic tool.
Based on ROC and Liu’s method, it was suggested that the best compromise between specificity and sensitivity to affirm the diagnosis of AKI is an increase in the AUC of at least 0.3mg/dl. With this threshold, the specificity (91.8%) is preferred over sensitivity (72.9%). Setting a threshold for a quantitative diagnostic test is always a trade-off between good sensitivity (with the risk of over-diagnosing and over-exposing patients) and good specificity (with the risk of under-diagnosing and not establishing early patient management).18
Based on our results, the threshold of 0.3mg/dl could increase true positives (n=52 or 83.9%) while the threshold of 1.5mg/dl gives true positives as high (n=28 or 93.9%). What is the potential clinical value of our findings? In the best case, an early increase in the level of uNGAL should be considered, but we find that sCr provides the same information to trigger a change in strategy in the clinical management of the patient. At a minimum, clinicians informed of such a situation should be aware of the potential for clinical AKI development. The increased difference in sCr values of even 0.3 mg/dl can make a significant contribution to clinical exploratory methods for prediction of early AKI. These patients would benefit from closer monitoring of blood pressure, urine output and renal infusion. All efforts to monitor the volume in postoperative and optimize hydration and renal infusion. These subjects should benefit from careful attention and control over the use of nephrotoxic products. The ability to predict which patients will develop AKI during hospitalization may allow early interventions to change the negative outcomes associated with their overly frequent clinical problem.
This study has some limitations. First, the study is monocentric and should be extended to several hospital structures and a large population. Second, it was noted that there was no core sCr in the general population and in our sample. In order to calculate the variation in the sCr for diagnosing AKI, we used the difference between the sCr at admission and the sCr at the 48-hour. Third, the diuresis of participants was not assessed in most patients. Urine collection from some participants was insufficient or there may be a lack of quality in urine collection. This could have an impact on the results of uNGAL, hence the need to use blood uNGAL.
The results of this study show that the sCr remains an effective tool for the diagnosis of early AKI in the context of resource-constrained countries. Patients with elevated sCr are at increased risk of adverse outcomes, including death and renal replacement therapy. Although our patients come from an urban hospital, our sample is made up of patients with different AKI etiologic factors and recruited from different departments.
None.
Authors declare that there is no conflicts of interest.
None.

©2022 Kahindo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.