eISSN: 2378-3176


Review Article Volume 5 Issue 2
1Department of Anaesthesia and Critical Care Medicine, Chitungwiza Central Hospital, Zimbabwe
2Faculty of Health and Science, University of Liverpool, UK
3Nephrology Department, Doncaster Royal Infirmary, UK
4Nephrology Department, Nottingham Children Hospital, UK
5Renal Transplant Department, Royal Liverpool University Hospital, UK
6Nephrology and Transplantation Department, Sheffield Teaching Hospitals, UK
Correspondence: Ajay Sharma, Renal Transplant Unit, Royal Liverpool University Hospital, University of Liverpool ? Prescot Street- L7 8XP- UK, Tel 44 7772362144, Fax 44 1517065819
Received: July 15, 2017 | Published: August 4, 2017
Citation: Chari M, Kosi ME, Jim JK, Sharma A, Halawa A (2017) Crossmatching in Renal Transplantation by Non-Immunologists for Non-Immunologists. Urol Nephrol Open Access J 5(2): 00166. DOI: 10.15406/unoaj.2017.05.00166
Before any kidney allograft transplantation is undertaken, crossmatching of prospective donor’s lymphocytes are performed against the prospective recipient’s sera to determine whether the recipient has any antibodies against the donor. Over the decades, the techniques of crossmatching have improved leaps and bounds from the complement-dependent cytotoxicity (CDC) test to the much more sensitive and specific Luminex single bead antigen assay. Nonetheless, CDC still remains the gold standard. An index case is, hereby, used to describe the pros and cons of the various techniques of crossmatching in kidney transplantation. The case based discussion in this article demonstrates that a transplant team may require a combination of more than one crossmatch techniques to assess an occasional complex puzzle that is posed to transplant clinicians when assessing patients with moderate to high immunological risks.
Keywords: human leukocyte antigen, donor-specific antibodies, crossmatching techniques, virtual crossmatch, kidney allograft, ımmunological risk
The major histocompatibility complex (MHC) genes are responsible developing a unique identity that is expressed in the form of a pattern of antigens that are expressed on donor cells, that are treated as ’foreign’ by the effect or system of immunological system of recipients leading to the rejection of mismatched kidney allografts. The MHC genes encode MHC antigens, which control the immune responses to foreign antigens.1 In humans, the MHC genes are called human leukocyte antigen (HLA) genes. The immunological barrier that is posed by HLA antigens is considered to be the main obstacle to kidney transplantation. HLA genes are found on the short arm of chromosome 6 where they are grouped into classes I, II and III. Class I genes include HLA-A, HLA-B and HLA-C, being expressed on all nucleated cells. Class II genes comprise HLA-DR, HLA-DP and HLA-DQ, and are expressed on antigen presenting cells. Presently, only HLA classes I and II are considered to be of significance in kidney transplantation. The determination of the donor’s and recipient’s HLA antigens is done using DNA (molecular) techniques. HLA typing ascertains the extent of the histocompatibility between the donor and recipient. The greater the HLA mismatch, the greater the risk of graft rejection. Therefore, HLA matching is crucial for stratifying the risk of immunological failure to kidney graft survival.2
Kidney transplantation between a donor and a recipient who have zero HLA mismatches were shown to have better graft outcomes compared to grafts with one or more HLA mismatches.3 United Network for Organ Sharing data for the period 1991 to 1997, showed a 11% difference in graft survival rate over three years between grafts with zero as opposed to six HLA mismatches.4 It was found that HLA-A, HLA-B and HLA-DR mismatches were associated with poorer patient and graft outcomes, with HLA-A mismatch having the least effect.5 Consequently, HLA typing for kidney transplantation has been employed as a routine for these three loci. Furthermore, the risk of acute kidney graft rejection was shown to be highest with mismatches at the HLA-DR position as opposed to mismatches at HLA-A and HLA-B positions.6 The influence of HLA mismatching on graft survival is depicted in (Figure 1).7 If the HLA typing result shows zero mismatching at HLA-A, HLA-B and HLA-DR loci, this is written as 0-0-0 mismatch, respectively.
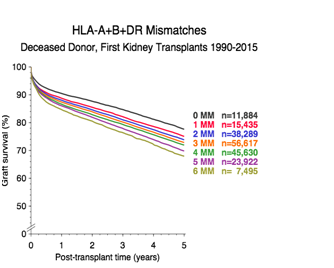
Figure 1 The influence of HLA mismatching on graft survival (www.ctstransplant.org/public/graphics/sample.shtml collaborative transplant study; k211010217).
Crossmatching techniques
After HLA typing, the recipient is tested for donor-specific antibodies (DSA). These are antibodies (Ab) against the HLA antigens of the prospective donor. If the DSA are detected, the recipient will then be deemed sensitized against this donor. DSA can be formed following pregnancy, blood transfusion, previous transplant and infections.8 If kidney transplantation is done in their presence, hyper-acute graft rejection may occur.9 Although HLA-C, HLA-DP and HLA-DQ matching is not routinely done, DSA against these antigens was associated with poorer kidney graft outcomes in retransplants to a varying degree.10,11 Crossmatching (XM) techniques to detect DSA include complement dependent cytotoxicity (CDC-XM), flow cytometry (FCXM), enzyme-linked immunosorbent assay (ELISA) and Luminex. CDC-XM and FCXM are cell-based, whereas ELISA and Luminex are solid phase immunoassays. Before discussing crossmatching in greater detail, it is worthwhile explaining what panel-reactive antibodies or, as more recently used, calculated panel-reactive antibodies mean.
Panel-reactive antibodies (PRAs) and calculated panel-reactive antibodies (cPRA)
PRAs are the anti-HLA antibodies that are detected during this crossmatch test. For example, on a 100-cell panel, a positive test against 80 donors means the PRA is 80%. It was used to estimate the percentage of donors who are incompatible with the recipient. Nowadays, PRA is of historical importance in kidney transplantation. Instead, calculated panel-reactive antibodies (cPRA) are used. A computerized calculator (algorithm) is used to determine the cPRA of the patient against a list of unacceptable HLA antigens found in the donor pool. The use of an algorithm means that cPRA is more consistent than PRA in assessing sensitization of recipients. The higher the cPRA, the less the chance of getting a compatible donor. As noted above, PRA values for HLA classes I and II are obtained separately. This contrasts with cPRA which uses one calculation for both classes.12 Thus, cPRA more accurately predicts the transplant risk of the recipient. Unlike PRA, cPRA is specific as to which HLA antigens the recipient is sensitized.
Immunoglobulin G (IgG) antibodies against HLA class I antigens are an absolute contraindication to kidney transplantation because they cause hyper-acute kidney rejection. They optimally interact with T lymphocytes at 37°C, whereas immunoglobulin M (IgM) antibodies react with T-lymphocytes at 4°C. A positive CDC-XM result can be due to autoantibodies, and these are usually IgM. Autoantibodies are found in autoimmune diseases like lupus nephritis. An auto-crossmatch (recipient serum tested against recipient lymphocytes) can be used to exclude the presence of autoantibodies. IgM autoantibodies are normally ignored in kidney transplantation.13 The influence of IgM antibodies on the crossmatch result can be eliminated by the following maneuvres:
Cell-based assays
Included under this category are CDC-XM and FCXM tests.
CDC-XM test: To perform the CDC-XM, live T and B lymphocytes are obtained from the donor’s peripheral blood and separated by density gradient centrifugation. The recipient’s serum is then mixed with the donor’s T and B cells contained in separate wells. These mixtures are incubated at 37°C for 60minutes. After that, complement is added and the mixtures are incubated for another 60minutes. A fluorescent dye like ethidium bromide is then added before a fluorescent microscope is used to assess cell lysis. T lymphocytes have HLA class I antigens whilst B lymphocytes have both HLA class I and class II antigens. If the recipient’s serum contains antibodies against any of these HLA antigens, the antibodies will target such lymphocytes. This will activate the complement via the classical pathway, causing cell lysis. The dead lymphocytes will take up the dye but viable ones won’t, as seen through the florescent microscope. The result is positive if there is dye uptake and negative if there is none. The percentage of lysed lymphocytes is then determined to scale the result as strongly, moderately or weakly positive.
The CDC-XM test can be improved by the addition of anti-human globulin (AHG). During this test, AHG is added to the mixture of recipient serum and donor lymphocytes prior to the addition of complement. AHG molecules bind to DSA which are attached to donor lymphocytes. This increases the number of complement receptors, amplifying cell lysis. The AHG CDC-XM is more sensitive than CDC-XM as non-cytotoxic and low titer antibodies can be detected. A CDC-XM positive for both T-lymphocyte and B-lymphocyte may mean: (a) the presence of anti-HLA class I DSA or (b) the presence of multiple anti-HLA class I with or without anti-HLA class II DSA. A CDC-XM negative for both T-lymphocyte and B-lymphocyte may occur: (a) when DSA against both HLA class I and II antigens are absent, (b) when non-complement-fixing DSA are present or (c) when the DSA titer is too low to cause cell lysis. A negative T-lymphocyte and a positive B-lymphocyte CDC-XM result may indicate: (a) the presence of anti-HLA class II DSA or (b) the presence of low level anti-HLA class I DSA. A positive T-lymphocyte and a negative B-lymphocyte CDC-XM may suggest a laboratory error. A repeat crossmatch should be done.
A positive T-lymphocyte CDC-XM in the absence of IgM autoantibodies is currently an absolute contraindication to kidney transplantation. One the other hand, the importance of a positive B-lymphocyte crossmatch should be interpreted together with the Luminex test.14 This is because as much as 50% of B-cell CDC-XM results can be false positive.15 Thus, a positive B-cell result is considered as a relative contraindication to transplantation. An advantage of performing CDC-XM test is that it detects complement-fixing antibodies, which are graft-damaging. The other is that transplant laboratories are experienced with its use. Some of the disadvantages of CDC-XM are that: (a) it cannot detect low antibody titers, (b) its accuracy is affected by viability of the lymphocytes, (c) it is not specific as to which antibody is causing the positive result, (d) it detects IgM autoantibodies and non-HLA antibodies and (e) inter-observer differences may occur. Figure 2 below illustrates the CDC-XM steps.
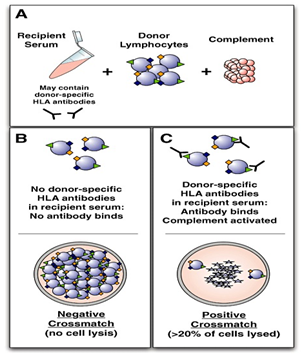
Figure 2 The CDC-XM technique flowchart [8]. Recipient serum potentially containing donor specific anti-HLA antibodies is added to donor T or B lymphocytes, along with complement (A). If donor-specific antibodies are not present, no lysis occurs and the result is deemed negative (B). If donor-specific anti-HLA antibodies bind to the lymphocytes and then activate complement, cell lysis will occur and the crossmatch result will be deemed positive (C). The proportion of lysed cells is assessed and the crossmatch is graded a being weakly, moderately or strongly positive.
(Curtesy: William R. Mulley, John Kanellis. Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology 2011; 16(2): 125-133.)
(B) FCXM: FCXM is similar to CDC-XM in that recipient serum and donor T- and B-lymphocytes are utilized as initial components. After 30 minutes of incubation at room temperature, the mixture is washed. Following this, AHG conjugated with fluorescent isothiocyanate is added to target any attached DSA. Incubation at 4°C for 15 minutes follows. Anti-CD3 or CD19 antibody conjugated with phycoerythrin (PE) is then added before incubation for another 15 minutes at 4°C. After washing, a flow cytometer is then used to measure the degree of fluorescence, which is recorded as channel shifts. FCXM is more specific than CDC-XM since it can detect specific DSA iso-types like IgG1, IgG2, and so on. It can also detect complement fixing as well as non-complement fixing antibodies. Additionally, FCXM detects IgG but not IgM antibodies. However, there is no standard channel shift value for a positive test among transplantation centers. A positive FCXM accompanied by a negative CDC-XM could indicate the presence of non-complement-fixing or low titer antibodies. Kidney transplantation in this scenario can result in antibody mediated rejection. A false positive result can be seen in patients on monoclonal antibody therapy such as rituximab.
Solid phase assays
These include ELISA and Luminex. Viable lymphocytes are not needed in solid phase immunoassays.
ELISA: ELISA uses pure HLA antigens fixed on polystyrene micro-titer plates into which the patient’s serum is added. Various commercial kit systems are available. They include sandwich direct, sandwich indirect, direct and indirect ELISA methods. Some of the kit contents are plates, detection and capture antibodies, wash solution, diluents, substrate, manual, stop solution and secondary antibody. ELISA is done in a defined order and under specific conditions as directed by the accompanying manufacturer leaflet. These steps include incubation periods and a series of washes. It is usually done post-transplant to quickly test for DSA. ELISA is more specific, more sensitive, faster and simpler to perform than CDC-XM and FCXM crossmatches.16 Compared to Luminex, ELISA is cheaper and faster to perform.
Luminex single-antigen bead (L-SAB)
The Luminex assay is now taken as the reference technique for DSA testing.17 It uses tiny polystyrene beads containing fluorochromes of different intensity with HLA antigen(s) immobilized on their surfaces. In L-SAB assay, each bead has a single HLA antigen attached to it. Thus, if the result is positive, L-SAB is very specific as to which DSA is present. This test can detect complement fixing and non-complement fixing antibodies, but not IgM autoantibodies or non-HLA antibodies. L-SAB is done by mixing the recipient’s serum with the beads and adding anti-human IgG labelled with PE. The mixture is then passed through two laser beams, and the result given as mean fluorescent intensity (MFI). MFI is the average fluorescence strength (intensity) obtained for each bead being scrutinized. It can be used to quantify DSA titer. The higher the MFI, the higher the DSA titer. Currently there is no uniformly agreed MFI value for a positive result among transplantation centers, with values differing from 1000 to 5000.18
The major advantages of L-SAB assay over other DSA detection tests like CDC-XM are that: (a) it is faster, more sensitive and specific (only anti-HLA antibodies are detected), (b) it utilizes smaller quantities of serum, (c) there is no need for viable lymphocytes since commercial kits are available, (d) it is reproducible, not affected by linkage disequilibrium, and (e) from the MFI value, the amount of DSA present can be determined, thereby, helping with the precise management of patients. Figure 3 below illustrate L-SAB test flowchart.8 Some of the drawbacks of L-SAB include: (a) higher cost, (b) false positive result from denatured antigens on beads (if present), (c) false positive result from non-HLA antibody binding, (d) false negative result when complement C1 and IgM antibodies bind to the beads, preventing HLA antigen-antibody interaction (prozone phenomenon), (e) false negative result from intravenous immunoglobulin interference, (f) false negative result if the HLA antigen on the bead is inaccessible, (g) false negative result if same epitope is shared by many beads, leading to a decrease in MFI, and (h) false negative result if a particular HLA antigen is not on the L-SAB bead kit.
The prozone phenomenon is usually seen in recipients with high sensitization levels. These patients have high antibody levels. In this case, a strongly positive CDC-XM and a negative L-SAB assay result may be seen. The prozone effect may be eliminated by diluting recipient serum. Adding DTT or ethylenediaminetetraacetic acid (EDTA), or heating the serum will have the same effect.19 Due to the very high sensitivity of L-SAB, situations where the L-SAB is positive but the CDC-XM and FCXM are negative may arise. It is, therefore, vital to consider results from both cell-based and L-SAB assays when assessing the transplant suitability of a patient. Table 1 below compares the various XM tests described above.
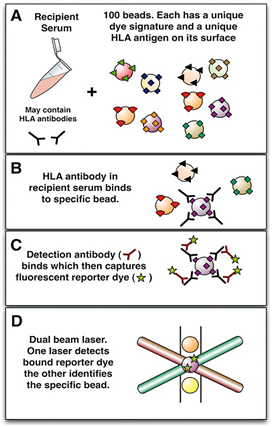
Figure 3 The virtual crossmatch. Recipient serum potentially containing anti-HLA antibodies is added to a mixture of synthetic beads. Each bead is coated with a set of antigens (screening beads) or for more precise detail, with a single antigen (single antigen beads). A unique dye signature (up to 100) specifies the identity of each bead (A). If anti-HLA antibodies are present these will bind to the appropriate bead (B) and a detection antibody can subsequently bind and capture a reporter dye (C). Each unique bead can then be interrogated for the presence of the reporter dye on its surface using a dual beam laser (D). A profile of antibodies can thus be identified in the recipient and compared with the known HLA identity of any potential donor, allowing a prediction of the crossmatch result.
(Curtesy: William R. Mulley, John Kanellis. Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology 2011; 16(2): 125-133.)
|
CDC |
FCXM |
ELISA |
L-SAB |
Phase |
Cell-based |
Cell-based |
Solid-phase |
Solid-phase |
Ab titer detected |
High |
Moderate |
Low |
Very low |
Complement-fixing Ab detected |
Yes |
Yes |
Yes |
Yes |
Non-complement-fixing Ab detected |
No |
Yes |
Yes |
Yes |
HLA Ab detected |
Yes |
Yes |
Yes |
Yes |
Non-HLA Ab detected |
Yes |
Yes |
No |
No |
IgM Detected |
Yes |
No |
No |
No |
Table 1 Comparison of various crossmatching techniques
Virtual crossmatch (vXM)
Virtual crossmatch involves comparison of recipient’s anti-HLA antibody profile, as detected by L-SAB assay, with the HLA antigens of the prospective donor. This is aimed to identify unacceptable donor HLA antigens. It is positive if DSA is present, and negative when absent. The recipients awaiting transplantation are tested for DSA every three months and every two weeks after a sensitizing event like blood transfusion. If the recipient is not sensitized against the donor, transplantation can be done without a final FCXM. This quickens the transplantation process and reduces costs. Other advantages of vXM are that acceptable recipients are quickly identified, cold ischemia time is reduced, unnecessary graft shipments are avoided and the chance that a recipient with high sensitization gets transplanted is increased. Frequent testing for DSA is one main prerequisite of vXM. The problem with vXM based on L-SAB is that it will show a false negative result if DSA against a rare allele is present (since rare alleles are not usually attached to the beads), which may result in graft loss. Given its high sensitivity, L-SAB can also detect clinically unimportant DSA, leading to some recipients missing the chance to be transplanted. Finally, the prediction of a positive FCXM by vXM is affected by the MFI value used and the DSA type.20
A thirty-year old male patient with stage 5 chronic kidney disease due to lupus nephritis. He has been on hemodialysis for the past 5 years with no history of blood transfusion or previous transplantation. He was offered a kidney allograft from a deceased donor. Laboratory investigations showed:
→ 1-0-0 HLA mismatch
→ CDCXM positive for both B and T cells
→ FCXM negative for both B and T cells
→L-SAB negative for DSA
Should this donor be accepted?
Case interpretation and management
This patient is a good kidney transplant candidate because: (a) he has no history of sensitization, (b) there is only one out of six HLA mismatch, (c) the mismatch is at HLA-A (d) L-SAB is negative for DSA, and (e) FCXM is negative for both T- and B-cells. He needs transplantation because he is young and has been on hemodialysis for a long time. Chronic hemodialysis carries a high mortality risk.21 The positive CDC-XM in this patient may be due to autoantibodies or a laboratory error. The patient has lupus nephritis, so autoantibodies are expected, since it’s an autoimmune disease. Luminex-SAB is negative for both anti-HLA class I and II DSA, pointing to the presence of autoantibodies. An auto-crossmatch should be done to confirm this before transplantation. The initial CDC-XM test should also be redone with DTT added. If the test becomes negative, then the autoantibodies are IgM and transplantation can proceed. In this case, the patient will be classified as standard risk.22 If it remains positive, then IgG alloantibodies will be the likely cause. This patient has a negative L-SAB, therefore, we infer that these IgG alloantibodies would be non-HLA. In that case, the patient can still be transplanted because this would be a standard risk scenario. Table 2 below shows some of the immunological risk levels in kidney transplantation.23 To rule out laboratory error, the quality of cells and other test conditions should be checked. In conclusion, this deceased donor kidney offer should be accepted. Transplantation should go ahead because the positive CDC-XM is due to autoantibodies.
Crossmatch type |
Outcome |
Risk Level |
CDC-XM |
Positive |
High |
CDC-XM |
Negative |
Intermediate |
FCXM |
Negative |
Standard |
CDC-XM and/or FCXM |
Positive |
Standard |
CDC-XM and/or FCXM |
T-cell positive, B-cell negative |
Standard |
Table 2 Immunological risk levels in kidney transplantation. (Curtesy: Callus R, Buttigieg J, Anastasi AA, Halawa A. Basic concepts in kidney transplant immunology
British Journal of Hospital Medicine 2017; 78 (1): 36).
Assessment and stratification of immunological risk of a prospective recipient requires interpretation of clinical background, types of crossmatch tests and cPRA. We need to be aware of comparative strength and limitations of these tests. The index case in this article is an immunologically low-risk patient with no history of sensitization, has positive B cell CDC cross-match, though with negative DSA by L-SAB. This CDCis false positive due to auto-immune disease i.e. lupus nephritis, there by demonstrating the importance of carefully interpreting available reports and understanding their limitations. Further testing to negate IgM and auto-antibodies has been suggested for the given case. In some patients with complex immunology, a careful and judicious use of a battery of immunology tests is of utmost importance to do a safe transplant and to avoid exclusion of a good donor.24
All authors declared there are no conflicts of interest.
None.
None.

©2017 Chari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.