eISSN: 2378-3176


Mini Review Volume 5 Issue 4
1Departamento de Nefrolog
2Departamento de Nefrolog
3Hospital Civil de Culiacan Sinaloa. Centro de Investigacion y Docencia en Ciencias de la Salud (CIDOCS), Mexico
Correspondence: Rafael Valdez-Ortiz, ISN Fellow 2010-2012. Head of Nephrology Service, Hospital General de Mexico, Dr Eduardo Liceaga, Mexico City. Dr Balmis No. 148 Col. Doctores, Delegación Cuauhtemoc CP 06726, Mexico, Tel 52 1-9991552275, Tel 52 (55) 2789 2000
Received: August 13, 2017 | Published: October 9, 2017
Citation: Geovana MA, Angeles ECMDL, Edgar DL, Rafael VO (2017) Benefits of Physical Exercise in Patients with Chronic Kidney Disease and Hemodialysis: A Mini Review. Urol Nephrol Open Access J 5(4): 00177. DOI: 10.15406/unoaj.2017.05.00177
Protein Energy Wasting (PEW) is characterized by abnormalities in skeletal muscle with a significant decrease in physical function and increased morbidity and mortality. Among all the factors associated with mortality of hemodialysis patients, impaired physical function is a factor that can be easily and directly modified by exercise. Different studies have shown that physical exercise significantly improves muscle strength and physical function. The implementation of a structured resistance exercise (RE) program during the hemodialysis sessions is a safe and effective intervention that helps to improve physical performance, nutritional status, quality of life, anabolic response, and muscle strength, being also effective to reduce inflammation and PEW. Chronic kidney disease is a catabolic state that activates different intracellular signaling pathways that decrease protein synthesis and increase protein degradation. There is enough evidence to establish the safety and benefits of performing exercise in patients on hemodialysis, however, there are other lines of research that are being developed to support this. It is necessary to continue evaluating exercise in prospective and long term studies. In conclusion, we must consider that patients on hemodialysis who perform physical activity are under a safe and non-pharmacological intervention that does not require an extra cost with promising results to prevent deleterious outcomes such as PEW, frailty and impaired physical function.
PEW, protein energy wasting; RE, resistance exercise; CKD, chronic kidney disease
It is well known that patients with chronic kidney disease (CKD) on hemodialysis have multiple metabolic and nutritional alterations that favor the development of a different type of malnutrition called protein energy wasting (PEW).1 PEW is characterized by abnormalities in skeletal muscle with a significant decrease in physical function and increased morbidity and mortality.2 Among all the factors associated with mortality of hemodialysis patients, impaired physical function is a factor that can be easily and directly modified by exercise (Figure 1). Different studies have shown that physical exercise significantly improves muscle strength and physical function.3-6 Physical function and functional limitations of hemodialysis patients can be measured by different tests such as six-minute-walk test, time-up-and-go test, gait-speed or sit-to-stand test.7. With these tests it has been shown that for every 0.1 m/s decrease in gait-speed, the risk of mortality increases by 26%, while a one-second increase in time-up-and-go test increases mortality by 8%.8 Physical function and muscle strength do not necessarily depend on muscle mass; some studies have reported that the first one is a better predictor of mortality in patients with CKD.9,10
A meta-analysis by Segura et al.11 concluded that intervention with resistance exercise (RE) have positive effects on different physical function tests, quality of life, lower-limb strength and body mass index. In this sense, RE has been reported to increase mitochondrial biogenesis, which is essential for maintaining the functionality and integrity of skeletal muscle.12 Johansen et al.13 showed how RE has anabolic effects. This study included patients on hemodialysis who received RE for 12 weeks. At the end of the study patients significantly increased fat mass, arm muscle area and muscle strength. One year later, in a review made by the same author, she concluded that exercise could also improve various physical performance tests, reduce blood pressure, and decrease systemic inflammation by improving endothelial cell function.14
On the other hand, aerobic exercise has been shown to improve the efficacy of mitochondrial oxidative metabolism of muscle15 and to increase the muscular mitochondrial volume.16 A meta-analysis by Smart et al.17 observed the effects of different types of exercise on the improvements of various physical fitness tests such as maximal oxygen consumption and muscle strength, they also observed improvements on energy intake and significant reductions in serum concentrations of C-reactive protein and IL-6, they conclude that physical training was shown to be a safe intervention with no deaths associated with exercise. Other studies have shown that the implementation of a structured RE program during hemodialysis sessions is a safe and effective intervention, that helps to improve physical performance, nutritional status, quality of life, anabolic response, and muscle strength, being also effective to reduce inflammation and PEW.18—20
On the other hand, long-term studies have documented how aerobic exercise after six months increases the cross-sectional area of muscle fibers by 46% and decreases the proportion of muscle atrophy in muscle fibers type I, IIa and IIx.21 In 2006, Workeneh et al.22 determined the impact of different types of exercise in the 14-kD actin fragment, which indicates the activation of muscle proteolysis. In this study the patients were randomized and assigned to four different exercise groups: 1) aerobic exercise (n=7); 2) anaerobic exercise (n=7); 3) aerobic and anaerobic exercise (n=6); 4) without exercise (n=8). After 18 weeks of exercise, it was found that aerobic exercise and the combination of aerobic and anaerobic reduced the concentrations of actin fragments, which suggests a decrease in muscle proteolysis, whereas with anaerobic exercise reduction in actin fragments it was minimal.
Chronic kidney disease is a catabolic state that activates different intracellular signaling pathways that decrease protein synthesis and increase protein degradation.23 Some of the pathological mechanisms described in the literature that favor this catabolic state involve metabolic acidosis, increase angiotensin II, systemic inflammation and a sedentary lifestyle.24,25 These conditions increase concentrations of myostatin, which in turn inactivates the signaling pathways mediated by the serine/threonine kinase mTOR and AKT-p. These increase the expression of the for head transcription factor (FoxO) that enters to the nucleus and activates the ubiquitin proteasome system and caspase-3 associated with the activation of apoptosis (Figure 2).
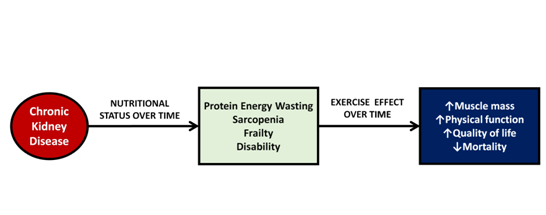
Figure 1 Effects of chronic kidney disease over time and impact of exercise on muscle mass. Chronic kidney disease triggers a series of abnormalities in skeletal muscle causing energy protein wasting, sarcopenia, frailty and disability, however, exercise can improve muscle mass and physical function and consequently improve nutritional status and quality of life.
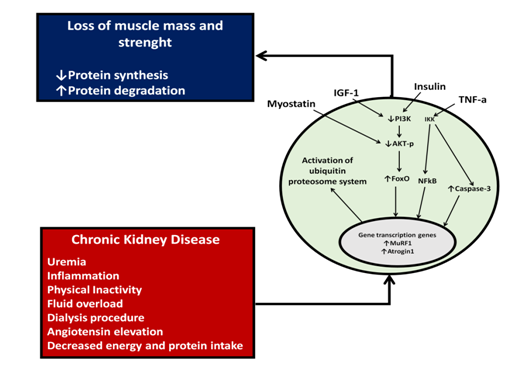
Figure 2 Cellular mechanisms involved in protein synthesis decrease and protein degradation increase in patients with chronic kidney disease. Complications associated with CKD through myostatin, IFG-1 (insulin-like growth factor), insulin and TGF-1 (transforming growth factor beta) activate intracellular signaling mechanisms that will decrease protein synthesis and increase catabolism.
There is enough evidence to establish the safety and benefits of performing exercise in patients on hemodialysis, however, there are other lines of research that are being developed to define this: What type of exercise is the best for hemodialysis patients?; Should exercise routines be intradialytic or interdialytic; Should exercise be accompanied by an adequate nutritional support?; What is the effect of exercise on the long-term follow-up in global and cardiovascular mortality?. It is necessary to continue evaluating exercise in prospective and long-term studies. We must consider that patients on hemodialysis who perform physical activity are under a safe and non-pharmacological intervention that does not require an extra cost with promising results to prevent deleterious outcomes such as PEW, frailty and impaired physical function.26
The authors declare that there are no conflicts of interest regarding the publication of this paper.
None.
None.

©2017 Geovana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.