eISSN: 2378-3176


Case Report Volume 4 Issue 1
1Central Laboratory Department, Prince Sultan Military Medical City, Saudi Arabia
2Pediatric Nephrology Department, Prince Sultan Military Medical City, Saudi Arabia
Correspondence: Majed Aloufi, Pediatric Nephrology Department , Prince Sultan Military Medical City, Riyadh, P.O Box 7897 Riyadh 11159, Saudi Arabia, Tel 00 966537110088
Received: March 01, 2017 | Published: January 17, 2017
Citation: Asiri S, Abdulmajeed N, Alzahrani S, et al. Early recurrence of focal segmental glomerulosclerosis after kidney transplantation in a child: a case report. Urol Nephrol Open Access J. 2017;4(1):12-14. DOI: 10.15406/unoaj.2017.04.00113
The survival rate of kidney graft after kidney transplantation has been improved following the introduction of current immune suppression drugs. However, the risk of recurrence or de novo glomerulonephritis still remains. Most forms of glomerular disease can recur after transplantation [1] but different forms of glomerulonephritis have widely different allograft outcomes. Whereas IgA nephritis recurs in up to one third of transplanted patients, this is not associated with adverse effects on graft survival. In contrast, recurrent focal segmental glomerulosclerosis (FSGS) has an unfavorable prognosis [2] and so early detection and appropriate treatment can be crucial. We are presenting here a patient with primary FSGS who developed acute kidney injury and heavy proteinuria in the first week post-transplant that responded completely to multiple plasmapheresis sessions and four doses of Rituximab.
FSGS: Focal Segmental Glomerulosclerosis; ATG: Anti-Thymocyte Globulin; EBV: Epstein-Barr Virus; PP: Plasmapheresis; BUN: Blood Urea Nitrogen
We present here in a case of an 11 year old girl with focal segmental glomerulosclerosis (FSGS) diagnosed at two years of age. She progressed to chronic kidney failure with peritoneal dialysis at 9 years. She underwent deceased kidney transplantation from an unrelated pediatric donor with a negative cross match with baseline characteristics of both donor and recipient are shown in Table 1. She was induced with anti-thymocyte globulin (ATG) and methylprednisolone and she started to pass urine immediately. Her serum creatinine decreased smoothly until it reached 105 micromol/L by day 3. Tacrolimus was introduced in the third day post transplantation and she was maintained on the triple immunosuppression agents of tacrolimus, mycophenolate and prednisone. Increasing body oedema, associated with more than 300 mg/l proteinuria by urine dipstick and a Protein/Creatinine ratio of 2500-4000 mg/mmol was noted on the third day post transplantation. Serum creatinine increased gradually over the subsequent three days to 324 micromol/L. Clinical examination confirmed hypertension with blood pressure about 135/85 mmHg, periorbital puffiness, distended abdomen with moderate ascites, and moderate lower limb oedema. At that stage, she was suspected to have a recurrence of her primary disease, so kidney allograft biopsy was urgently arranged.
|
Donor |
Recipient |
Age (year) |
10 |
11 |
Sex |
F |
F |
Weight (kg) |
30 |
24 |
Height(cm) |
138 |
127 |
Blood Group |
A+ |
A+ |
Tissue Typing |
A2; B8,51; CW4,15; DR1,7; DQ2,5 |
A32; B18; CW5; DR4,7; DQ2 |
CMV |
IgG positive |
IgG positive |
EBV |
IgG positive |
IgG positive |
Table 1: Characteristics of both donor and recipient.
Light microscopy: Sections stained for H+E, PAS, and Jones methenamine silver and trichrome stains showed the renal cortex containing 32 glomeruli with no segmental or global sclerosis. The glomeruli were normocellular and the capillary loops were not thickened. Interstitial fibrosis, inflammation and tubular atrophy were absent. The arteries and arterioles were unremarkable (Figure1).
Immunohistochemistry: Peritubular capillary staining for C4d was negative.
Immunofluorescence microscopy: Sections stained for IgG, IgA, IgM, C3, C1q, and fibrinogen. There was mild granular mesangial staining for IgM. All other stains were negative.
Electron microscopy: Sections stained with Toluidine blue stain showed the renal cortex containing 16 glomeruli, none globally sclerotic. Ultrastructure evaluation showed diffuse epithelial foot processes effacement with microvillus proliferation, mild amounts of electron dense deposits in the mesangial region. The glomerular basement membranes were normal thickness (Figure 2&3).
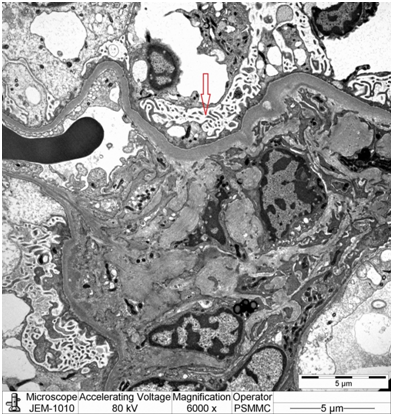
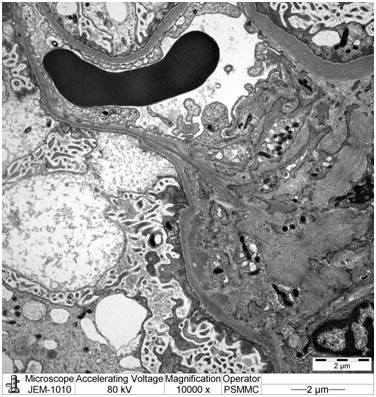
Diagnosis: Minimal change nephropathy indicating early recurrence of focal segmental glomerulosclerosis.
Plasmapheresis (PP) was started with albumin/fresh frozen plasma as replacement fluid for the initial intensive 12 sessions. Renal function recovered, edema resolved and creatinine levels dropped to 95 micromol/l. The patient was maintained on two sessions per week of PP, but continued to show heavy proteinuria. At that stage, we decided to give her four doses of Rituximab (375 mg/m2) that were given every other week without complication. Both enalapril and candesartan were added as antiproteinuric and antihypertensive agents. She was discharged home on once weekly PP with her blood urea nitrogen (BUN) 3.4 mmol/l, serum creatinine 48 micromol/L with protein/creatinine ratio between 800-1200 mg/mmol and stable graft and patient status. Her renal parameters continued to be within acceptable range and her protein/creatinine ratio gradually decreased over the subsequent six weeks after Rituximab, until it reached and stabilized at a level far below the nephrotic range proteinuria between 70-120 mg/mmol. So PP was discontinued after which she continued to have stable graft function without signs of recurrence of her proteinuria and FSGS.
Ten months later, she had another recurrence of her FSGS, with increased urine protein/creatinine ratio reached more than 1000 mg/mmol, and associated with slight increments in her serum creatinine, for which two doses of Rituximab given. She showed immediate recovery with normalization of Urine protein/creatinine ratio. Currently, she is more than three years after kidney transplantation with no more recurrence and latest serum creatinine is 52 µmol/L and urine protein/creatinine Ratio is 0.5 mg/mmol, she had no further admission since then. She had never diagnosed to have any infectious complications and her regular viral monitoring for Cytomegalovirus, Epstein–Barr virus (EBV) and BKV were unremarkable.
Focal segmental glomerulosclerosis is a common cause of end stage renal disease requiring renal transplantation [3]. In the pediatric age group, FSGS has a high rate of recurrence (up to 30%) [4-6]. The risk of recurrence in the first graft is high, up to 30%, with graft loss of 40-60% [4,7] while the risk of recurrence in a second graft is 60–100% [8]. Risk factors include: Onset of nephrotic syndrome during childhood, rapid course to ESRD (<3 years), female sex, bilateral nephrectomy, mesangial hypercellularity, presence of FSGS circulating factor [4,5]. The podocin mutation may play a role in pathogenesis and early recurrence of FSGS [3,9]. The presentation of recurrence includes early massive proteinuria (78% during the first month after transplant, often as early as in the first urine from the graft) and sometimes graft failure and hypertension [9]. Proteinuria may herald the development of FSGS even if an early biopsy does not show glomerular abnormalities under light microscopy (as our case). In other words light microscopy will not show any evidence for FSGS and only the presence of diffuse epithelial foot process effacement on electron microscopic examination can prove the diagnosis pathologically.
Patients with post-transplant proteinuria >2 g/day who have FSGS as their original disease should be treated as soon as possible with an intensive course of plasmapheresis [3]. In spite of the risk of recurrence, patients with FSGS should not be excluded from transplantation. In the case of living donation, the possibility of recurrence and its consequences should be clearly highlighted and discussed with the donor and the recipient and preemptive plasma exchange should be planned [3]. In this case, we believe that management was started sufficiently early and aggressively in order to prevent any negative outcome for her new transplanted kidney. The benefit of Rituximab has been suggested in multiple case series and case reports [10-19]. The number of doses given varies in different studies between 1-6 doses, with an overall success rate of up to 64%. Therefore, we decided on a course of four doses every other week. This achieved full remission after 6 weeks, which is the average response time reported in many studies.
This 11 years old girl with primary FSGS and early recurrence FSGS post transplantation was managed with a combination of multiple sessions of PP and four doses of Rituximab, after which she had complete remission. In conclusion, this is another case of recurrent FSGS that shows a good response to the combination of PP and Rituximab. Further longitudinal prospective controlled studies are needed in order to assess the efficacy of such a protocol in the management of recurrent FSGS.

©2017 Asiri, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.