eISSN: 2378-3176


Case Report Volume 1 Issue 2
Urology Department, Hospital Universitario Fundacion Jimenez Diaz, Spain
Correspondence: Simon Rodriguez C, Urology Department, Hospital Universitario Fundacion Jimenez Diaz, Avda Reyes Catolicos 2. 28040 Madrid, Spain, Tel 34-653961817, Fax 34-915504800
Received: October 24, 2014 | Published: November 29, 2014
Citation: Simon Rodriguez C, Cabello Benavente R, Quicios Dorado C, et al. Laparoscopic en bloc resection of the urachus and bladder dome in an urachus adenocarcinoma. Urol Nephrol Open Access J. 2014;1(2):66-68. DOI: 10.15406/unoaj.2014.01.00015
Urachal carcinoma is a rare malignancy with a poor prognosis. Surgical options for treating urachal adenocarcima include radical cystectomy and an en bloc partial cystectomy with excision of the urachus and umbilectomy. Laparoscopic approach is a less invasive alternative to open partial cystectomy. We report a 42years old man case with a history of 12years of mucinuria. Clinical investigations reveal an 11x5, 3x5cm size supravesical midline cyst. We performed a laparoscopic resection of the mass. The procedure was successful with an en bloc resection of the mass. Histological examination reveals a well differentiated mucine tubulo-papilar adenocarcinoma. Surgical margins were negative. No local or distant recurrences were observed at three years follow up.
Keywords: carcinoma, laparoscopy, mucinuria, urachus
US, ultra sonography; MR, magnetic resonance; CT, computer tomography; UA, urinalysis
The urachus is a vestigial structure that develops during embryogenesis from two structures: the cloaca, which is the cephalic extension of the urogenital sinus (a precursor of the fetal bladder), and the allantois, which is a derivative of the yolk sac. During the 4th and 5th month of normal fetal development when the bladder descents into the pelvis, the lumen of the allantois are obliterated, forming the urachus. This structure finally closes by the third trimester of gestation. In adulthood, the urachus is formed by a fibrous band that measures from 5cm to 10cm in length and from 8 to 10mm in diameter, also known as the median umbilical ligament, which extends upward the anterior dome of the bladder toward the umbilicus. It coalesces with the obliterates umbilical arteries to form the ligamentum commune. It is located extraperitoneally in the Retzius space, and is bounded by the transverse fascia ventrally and the parietal peritoneum dorsally. The persistence of an embryonic urachal remnant can give rise to various clinical problems, not only in infants and children but also in adults. An urachal carcinoma is an uncommon tumor that accounts for less than 1% of all bladder tumors.1–8 Surgical options for treating urachal adenocarcima include radical cystectomy and en bloc partial cystectomy with excision of the urachus and umbilectomy. In the era of minimally invasive surgery, it seems reasonable to consider the laparoscopic approach for partial cystectomy.
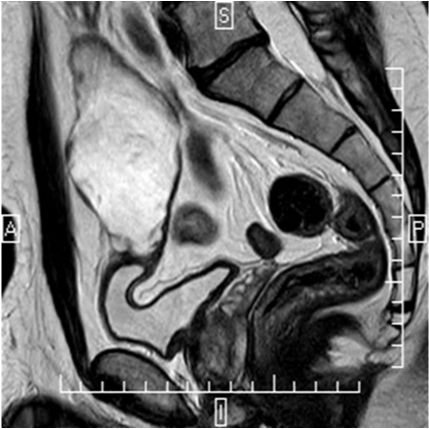
A 42-year-old man presenting a history of 12years of mucinuria. Physical examination revealed no abnormalities. Urinalysis, urine cytology and urine culture were normal. Cystoscopy revealed over the dome of the bladder a nipple shaped lesion covered by mucus. The trans abdominal ultrasonography (US) showed a 10cm cystic mass located above the superior bladder wall. Pelvis magnetic resonance (MR) imaging showed a 11x5, 3x5cm size supravesical midline cyst (Figure 1). No calcifications were observed. The clinical diagnosis was a urachal cyst. The patient underwent a laparoscopic en bloc urachus and bladder dome resection. After a urethral catheterization using 20-F 3 lumen catheters, in 15-30º Trendelenburg position, a longitudinal skin incision above the umbilicus was made to introduce a 12mm Hasson trocar for the camera port. A laparoscopy was performed to rule the presence of peritoneal metastases and the urachal tumor visualization. After both 10mm and 5mm working ports, placed on the left and right umbilicus line were inserted, an additional 5mm assistant port was placed through the left iliac fossa.
The dissection started from the cranial part of the tumor. The mass was separated from the abdominal wall using an electro thermal bipolar vessel sealing system (Ligasure, Covidien®) exposing the abdominal muscles. Afterwards a circumferential incision of the bladder 2cm away from the junction of the tumor was made using monopolar electrocautery. The bladder was reconstructed with a manual continuous transverse absorbable suture and inflated with saline solution to confirm watertight closure. The specimen was placed into a laparoscopic retrieval bag and extracted throughout the 12mm optical port wound, and Jackson-Pratt drainage was placed throughout the additional 5mm assistant working port. Finally, the laparoscope was reinserted to check a correct hemostasia. The abdominal wall was closed with a polydiaxanone suture in layers. The urethral catheter was left for 2weeks. Histological examinations confirmed a well differentiated mucine tubulo-papilar adenocarcinoma. Surgical margins were negative (Figure 2). Because this malignant neoplasm was found, afterwards a pelvic lymphadenectomy was made to check for lymph node invasion. Histological analysis of the ganglia did not reveal metastasis. The cancer stage classification according to Sheldom score was IIIA8 and stage II according to Mayo score4 (Table 1) At three years follow-up with CT scan, neither local recurrence nor distant metastasis were found.
Sheldom staging system |
Mayo staging system |
||
Stage I |
Urachal cancer confined to urachal mucosa |
Stage I |
Tumors confined to the urachus |
Stage II |
Urachal cancer with invasion confined to urachus itself |
Stage II |
Tumors extending beyond the muscular layer of the urachus and/or the bladder |
Stage IIIA |
Local urachal cancer extension to bladder |
Stage III |
Tumors infiltrating the regional lymph nodes |
Stage IIIB |
Local urachal cancer extension to abdominal wall |
Stage IIIB |
Tumors infiltrating non regional lymph nodes or other distant sites |
Stage IIIC |
Local urachal cancer extension to peritoneum |
||
Stage IIID |
Local urachal cancer extension to viscera other than bladder |
||
Stage IVA |
Metastatic urachal cancer to lymph nodes |
||
Stage IVB |
Metastatic urachal cancer to distant sites |
||
Table 1 Urachal cancer staging system as defined by sheldom and mayo staging system
Congenital urachal anomalies are twice as common in men as in women. The most common congenital urachal anomalies are a patent urachus that accounts for about 50% of all cases of congenital anomalies, urachal cysts (about 30%), vesicourachal diverticulum (about 3-5%) and a patent urachus with communications between the bladder and umbilicus (about 15%). The majority of patients with urachal abnormalities (except those with a patent urachus) are asymptomatic. Benign urachal neoplasm including adenomas, fibromas, fibroadenomas, fibromyomas, and hamartomas are extremely rare; however, they are important in that they mimic urachal malignancy.7 Malignant urachal neoplasms are also rare.8 Although the normal urachus is most commonly lined by the transitional epithelium, most urachal malignant tumors are adenocarcinomas that counts for 90% of case; conversely 34% of bladder adenocarcinomas are urachal origin.3 Other histologic subtypes like sarcomatoid, squamous or transitional cell elements have been reported.
Although the lumens of urachal remnants passing through the bladder wall are most frequently covered with normal appearing transitional cell epithelium, urachal cancers are nearly always adenocarcinomas. The origin of these adenocarcinomas is not definitely known. They display an enteric-type histology more frequently associated with colorectal cancer. These tumors often have glandular structures and mucin production, and may also have colloid and/or signet-ring cell histology present. They are believed to originate either from enteric rests left behind from the cloaca during embryological development or from a metaplasia of the urachal mucosa into columnar epithelium, followed by a malignant transformation.1–3 Because urachal cancers typically show an enteric type pastern, urachal adenocarcinoma may express detectable serum levels of carcinoembryonic antigen (CEA), CA125 and (CA) 19-9. In one series these markers were elevated in 40-60% of patients. Urachal adenocarcinomas tend to have a male predilection. Typically occurs in a younger patient population with a median age at diagnosis is approximately 47-56years.1
Although the lumens of urachal remnants passing through the bladder wall are most frequently covered with normal appearing transitional cell epithelium, urachal cancers are nearly always adenocarcinomas. The origin of these adenocarcinomas is not definitely known. They display an enteric-type histology more frequently associated with colorectal cancer. These tumors often have glandular structures and mucin production, and may also have colloid and/or signet-ring cell histology present. They are believed to originate either from enteric rests left behind from the cloaca during embryological development or from a metaplasia of the urachal mucosa into columnar epithelium, followed by a malignant transformation.1–3 Because urachal cancers typically show an enteric type pastern, urachal adenocarcinoma may express detectable serum levels of carcinoembryonic antigen (CEA), CA125 and (CA) 19-9. In one series these markers were elevated in 40-60% of patients. Urachal adenocarcinomas tend to have a male predilection. Typically occurs in a younger patient population with a median age at diagnosis is approximately 47-56years.1 Patients with urachal cancer may have no symptoms until the later stage when sufficient growth has occurred to penetrate the bladder.1 It presents unfortunately with a locally advanced disease, usually with local invasion or metastatic disease and is not reliably cured with surgery.6 The most common clinical feature is hematuria. Other signs and symptoms are dysuria, abdominal pain, voiding of mucous-like material from the mucinous enteric elements seen on histopathology, or umbilical discharge. Physical examination should include an abdominal exam with palpation of the umbilicous, suprapubic area and lymph node palpation.2
A typical work-up includes cystoscopy with biopsy and radiographic evaluation.2 US, CT, and MR imaging have the ability to display cross-sectional images and therefore are ideally suited for demonstrating urachal anomalies. US may demonstrate a midline fluid-filled cavity with mixed echogenicity and calcifications adjacent to the anterior abdominal wall. A characteristic CT feature of urachal carcinoma is a midline mass anterosuperior to the dome of the bladder with low-attenuation components, which represent pools of mucin at pathologic examination. As with some other mucinous adenocarcinomas of the abdominal organs, urachal carcinomas may produce typical psammomatous calcifications that are well depicted at CT in 50%–70% of cases.7 Magnetic resonance imaging is an excellent staging tool. Because of the presence of mucin within the tumor, increased signal intensity is seen on T2-weighted spin-echo MR images. Both CT and MR imaging are useful for demonstrating intra- and extravesical extension of the tumor.3
A broad staging system was initially proposed by Sheldon.8 Recently, investigators at the Mayo Clinic (MN, USA) proposed an alternative and more simplistic staging system4 (Table 1). An early complete resection of the tumor remains the mainstay of treatment. Survival in cystectomy/umbilectomy or in an en bloc radical cystoprostatectomy/umbilectomy has comparable outcomes. Thus in the initial management of urachal carcinoma, umbilectomy with partial cystectomy may be considered in selected cases.9 Partial cystectomy cures 70% of patients with clinically localized urachal cancers.6
Wadhwa et al.,10 reported the first series of three cases of laparoscopic partial cystectomy using a transperitoneal approach. The anatomic boundaries of resection that they used included resection of the tumor, with macroscopically normal bladder margins forming the distal limit; extensive resection of the peritoneum lateral to the two medial umbilical ligaments, which defined the lateral limits, the posterior sheath of the rectus muscle of the abdomen of the arcuate line and the muscle fibers of the rectus muscle below it, and the extra peritoneal fat in the space of Retzius as the anterior limit; and the urachus up to the umbilicus superiorly, sparing the umbilical skin. No recurrence was observed at the median follow-up of 6, 5months.
Other techniques had been reported. Porpiglia et al.,11 combined an endoscopic and laparoscopic resection of urachal carcinoma. Cystoscopic evaluation enabled to perform biopsies at the four cardinal points of the lesion; after confirmed that the frozen sections prove negative the laparoscopic procedure commences starting at the point of crossover of the vas deferens and continuing posteriorly around the bladder, following through towards the umbilicus. The peritoneum, umbilical ligaments and urachus were dissected. Cystoscopy and rigid forceps are used to ensure precise incision on the bladder dome. Through an endo-GIA vascular stapler, the bladder dome is sectioned along the traced line. The lesion is extracted in a closed state in a lap sac, avoiding dispersal of neoplastic cells.
Tai et al.,12 suggested another method of laparoscopic partial cystectomy, with excision and pushing the umbilicus into the peritoneal cavity prior to laparoscopy. An Endo-GIA stapler was used for excision of the tumor and bladder dome. No consensus is about to perform a lymphadectectomy and its limits. But it is reasonable to make a pelvic lymphadenectomy to asses a lymph node invasion, and offer an adjuvant treatment. Currently, there are no standard recommendations regarding adjuvant or neoadjuvant chemotherapy or radiation therapy in the treatment of urachal tumors.1 It is unclear whether chemotherapy or radiation treatment is of any benefit to patients with urachal carcinoma. However its similarity to colorrectal carcinoma which is responsive to perioperative 5-fluorouracil seems to be reasonable to offer chemotherapy to patients at high risk of prolapse. The role of radiation therapy is unclear.
Urachal adenocarcinoma is a rare form of cancer with poor a prognosis. Surgery remains the main step of therapy. Laparoscopic partial cystectomy is a safe and feasible treatment in selected cases with clinically localized urachal adenocarcinomas. However, long-term follow-up in larger series is needed to finally determine the role of laparoscopy in treating the disease.
None.
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.

©2014 Simon, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.