eISSN: 2577-8285


Case Report Volume 4 Issue 2
1Department of Neurology, Mayanei Hayeshua Medical Center, Bnie Barak and the Sackler Faculty of Medicine, Tel-Aviv University, Israel
1Department of Surgery, Tulane University, USA
2 Department of Pediatrics, Mayanei Hayeshua Medical Center, Bnie Barak and the Sackler Faculty of Medicine, Tel-Aviv University, Israel
2Department of Radiology, Tulane University, USA
3 Department of Radiology, Mayanei Hayeshua Medical Center, Bnie Barak and the Sackler Faculty of Medicine, Tel-Aviv University, Israel
Correspondence: N Gadoth MD, Dept of Neurology, Maynei Hayeshua Med Ctr. 17, Povarsky St. Bnei Barak, Israel, Tel 97235775455, Fax +97235775454, Tel 412-607-3047
Received: May 11, 2020 | Published: May 25, 2020
Citation: Gadoth N, Assalia N, Zeltzer Y. Sleep spindles in infantile stroke.Sleep Med Dis Int J. 2020;4(2):32?36. DOI: 10.15406/smdij.2020.04.00069
Background: Significant amplitude suppression of sleep spindles (SS) was observed in EEG of adults with stroke. Those changes were present in most patients on the side of the stroke. However, there are no data on the effect of stroke on spindles in infants and children.
Methods: We report two infants with hemispheric ischemic stroke in whom marked amplitude depression or absence of spindles occurred on the affected side. We also provide a literature review on the association of stroke and several neurodevelopmental disorders with spindle abnormalities.
Results: Both cases of infantile acute hemispheric stroke were 8 and 9 months old. Scalp EEG disclosed absence or marked amplitude depression of spindles in the ipsilateral side of the stroke in case 1 and 2 respectively.
Conclusions: The effect of hemispheric stroke on spindles in both infants and adults is similar. It is conceivable that such changes may affect the quality of non-REM sleep and as a result impair daytime alertness already documented in adults with stroke and children with a variety of neurodevelopmental disorders.
Keywords: sleep spindles, EEG, stroke, thalamus, infancy, endocarditis
SS, sleep spindles; EEG, electroencephalography; nREM, non rapid eye movement sleep; CVA, cerebrovascular accident; CRP, C-reactive protein; PCR; polymerase chain reaction; AST, Aspartate Aminotransferase; AT, alanine transaminase; PMN, polymorphonuclears; ESR, erythrocyte sedimentation rate; CSF, cerebrospinal fluid; WBC, white blood cells; RBC, red blood cells
Sleep spindles (SS), first described in detail by Loomis et al.,1 in 1935 are rhythmic bursts recorded routinely by scalp electroencephalography (EEG) during non-REM (nREM) sleep in normal subjects. The descriptive term “Spindles” referrers to the shape of each burst, which starts and ends abruptly with the lowest amplitude waves while maximal amplitude is seen at about the midpoint of the burst. All bursts share a constant frequency, typically between 12-14Hz. In youth and adults, spindle bursts of 0.5-2 second duration are recorded during light sleep at the vertex leads with a frequency of 13-14Hz. During deep sleep, their frequency becomes somewhat slower and their location is displaced frontally. SS can be recognized as early as at 2 months of age. During infancy SS appear in the frontal regions as arciform, usually unilateral, asynchronous bursts with up to 10 seconds duration reoccurring every 10 seconds. During the second year of life, there is a transition to sinusoidal synchronous, centrally located shorter bursts. The presence of asynchronous bursts after 2 years of age is considered abnormal.2 Neurophysiology research points to the GABAergic thalamic reticular neurons as the main generator of SS.3 Thus, damage to thalamocortical network may affect the appearance of SS in the EEG recording as well as impair sleep quality. Indeed, Cross and Gibbs were the first to report in 1948 the presence of depressed spindle activity ipsilateral to hemispheric damage (mainly vascular) in 30 out of 31 adults.4 Similar EEG findings were seen following frontal lobotomy.5,6 Pediatric Cerebrovascular accident (CVA) referred hereby as “stroke”, is not rare. Annualized pediatric stroke incidence rates, including neonatal and later childhood ischemic and hemorrhagic strokes range from 3 to 25 per 100 000 children in developed countries.7 Unfortunately we could not find descriptions of sleep EEG in such patients. The purpose of this report is to describe in some details two infants with acute hemispheric stroke in whom complete absence or markedly depressed SS on the affected side was detected by scalp EEG. A review of the literature regarding the association of spindle shape, frequency and distribution with several neurodevelopmental disorders in children is provided.
Case 1
A 9-month previously healthy male was seen in our pediatric Emergency Department (ER) for acute left sided weakness. The parents reported that for the last 3 days their child suffered from fever accompanied by mild respiratory distress. About three hours prior to admission (PTA), he woke up crying and while being cradled the father noticed that his son's left eye deviated to the left. In the ER he was alert with normal eye movements and decreased use of his left limbs. Rectal temperature was 37.9°C and the neck was supple. The complete physical examination was normal except for a stuffy nose, congested conjunctiva and frequent productive cough. The neurological examination revealed normal cranial nerve function (Nerves 2-7, 9-11),decreased voluntary movements on the left with intermittent left sided fisting, moderate left side weakness with decreased muscle tone involving arm more than leg, increased deep tendon reflexes, more so of the left hand , and bilateral flexor plantar responses. Withdrawal from pin prick was considered as normal sensation to pain. There was no family history of chronic neurological disorders, bleeding diathesis, thromboembolic events and presence of recent infection in other five siblings and in his nursery school. There was no history of recent trauma or exposure to drugs or toxins. Initial laboratory investigation revealed an erythrocyte sedimentation rate (ESR) of 41 mm/h, and C-reactive protein (CRP) of 25mg/dl (normal range 0.05-5mg/dl). Complete blood counts and blood chemistry were unremarkable. Lumbar puncture disclosed clear sterile fluid under normal opening pressure with 2 White Blood Cells (WBCs)/mm3 and 2 Red Blood Cells (RBCs)/mm3. Polymerase Chain Reaction (PCR) for human adenovirus and enterovirus from a nasal swab specimen were positive. Herpes simplex 1; 2, varicella zoster, influenza type A; B, respiratory syncytial virus and enterovirus were negative. Coagulation function series were unremarkable except for D-dimer of 701 ng/ml (normal range 0-500 ng/ml) Protein S and Factor V Leiden was normal while protein C was 61% (normal 80-150%) Echocardiogram was normal. Brain MRI (Figure 1) disclosed ischemic changes in the right lenticulostriate artery territory compatible with acute CVA. EEG disclosed total absence of SS on the right during nREM sleep throughout the entire tracing (Figure 2). Ceftriaxone, acyclovir, metronidazole and aspirin were initially administered and maintained for several days until cultures returned negative. The left side weakness slightly improved to the extent that his pinch returned to normal. On hospital day 3 a short focal tonic-clonic seizure involving the left arm and leg accompanied by left gaze deviation with subsequent left central facial palsy was observed for which levetiracetam (KeppraR) was started. The parents were reluctant to show up for clinical, EEG and MRI follow up. They were contacted by phone three months later and reported that the left sided weakness has completely resolved.
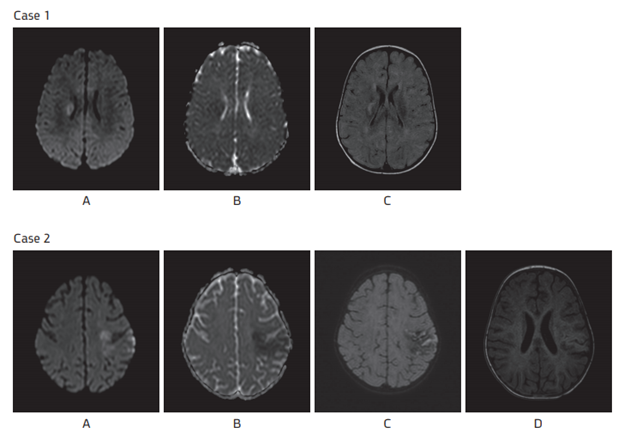
Figure 1 Brain MRI.
Case 1: Diffusion weighed imaging showing high signal intensity (A) with restriction on ADC map (B) and high signal intensity on T2 Flair weighted imaging (C)
Case 2: Diffusion weighted imaging showing high signal intensity (A) with restriction on ADC map (B) gyral high signal intensity on T2 Flair weighted imaging (C) associated with low signal T2 and high signal T1 (D) corresponding to cortical laminar necrosis.
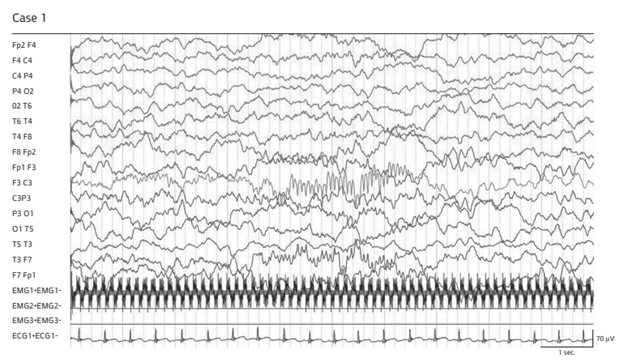
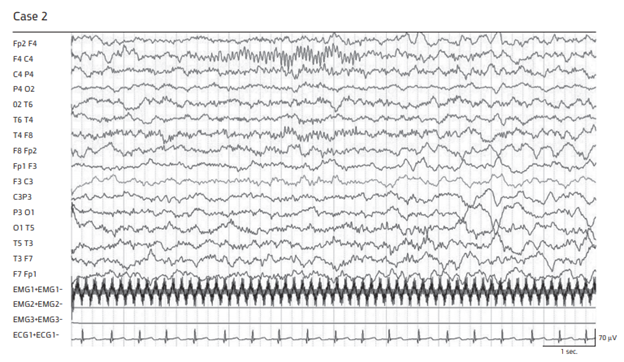
Figure 2 Scalp EEG during spontaneous nREM sleep.
Note absent spindles on the affected side in case 1 and markedly depressed spindles in case 2.
Case 2
This 8-month-old female was admitted to our pediatric ER after 4 days of febrile illness accompanied by persistent vomiting. On admission rectal temperature was 38.4°C with otherwise normal vital signs. She was well nourished and fully alert. The general physical examination disclosed a bulging anterior fontanelle while the rest of the physical examination was unremarkable except for discrete regions of maculopapular rash over the abdomen and lower extremities. Neurological examination was normal. Perinatal, postnatal and family history was non-contributory. WBC count of 30000/µL (20000 absolute neutrophils count), elevated Aspartate Aminotransferase (AST), Alanine Transaminase (ALT), and CRP were found. Lumbar puncture disclosed crystal clear fluid with 243/µL WBCs, of which 80% were polymorphonuclears (PMN), normal glucose and protein without initial bacterial growth. A combination of ceftriaxone and acyclovir were initiated. On day 4, Kingella kingae was isolated from blood culture while cerebrospinal fluid (CSF) culture was negative. Prompt echocardiography revealed mitral and tricuspid valve vegetations without mitral regurgitation. On the same day right hemiparesis and few left focal seizures were noted. Brain MRI revealed a large left frontal ischemic area extending to the basal ganglia consistent with subacute CVA in the territory of the left middle cerebral artery (Figure 1). On repeated neurological evaluation she was alert with right facial palsy. Spontaneous motor activity was missing on the right. Right upper extremity paralysis and lower extremity paresis, mainly distal, with mild increased muscle tone, hyperactive deep tendon reflexes, extensor plantar response and absent superficial abdominal reflexes were present. The Parachute reflex was present on the left side only. EEG disclosed disorganized high voltage delta activity in the left parieto-temporal leads and very low amplitude SS in the left central leads, which persisted throughout the whole tracing (Figure 2). On day 10, marked clinical and laboratory improvement was noted. Repeated echocardiogram revealed decrease in vegetation size. She was discharged on day 14 and continued intravenous antibiotic therapy for 5 weeks. On periodic outpatient clinic visits, gradual improvement of motor functions was noted. Repeated EEG was identical to the initial tracing.
In case 1, acute hemiplegia followed a febrile illness with symptoms of upper respiratory tract infection. Indeed, among the known risk factors for ischemic stroke in children (vasculopathy, trauma, cardiac and hematological), infection and especially upper respiratory infection, is a significant risk factor.8 Case 2 is an example of stroke caused by septic emboli secondary to Kingella Kingae endocarditis. This Gram-negative, anaerobic bacterium has been increasingly recognized as an invasive pathogen in childhood endocarditis. In a review of the literature until 2014, Foster et al.,9 were able to detect 39 children with bacteriologically proven Kingella endocarditis and added 3 personal cases. Out of those, 42 (77%), suffered from stroke.
In both of our patients the characteristic EEG finding obtained during the acute/subacute phase of stroke was a marked amplitude suppression of SS at the affected hemisphere to the extent that spindles could not be detected in case 1 and were markedly suppressed in case 2. To note that in this case a repeated EEG obtained 3 weeks after the initial recording was identical to the first tracing. Although the thalamus is the source of spindle oscillations, their synchronization over a wide area of the thalamus and cortico-cortical network is dependent on cortical feedback to the thalamus and cortico-cortical connection.10 Published data on the relation between hemispheric stroke and SS and its impact on sleep quality and daytime alertness are available only for adult patients in whom the stroke involved the anatomic neural network mentioned above. The abnormalities of spindles are not uniform and range from total absence or slowing of spindle oscillations ipsilateral to the lesion,11,12 bilateral distribution in patients with large strokes,13 and decreased frequency of spindles on the affected side.14 In patients with left paramedian thalamic stroke, reduction in individual spindle power was found.15
Published data on abnormalities of SS in children are scarce and of limited values since most studies on that topic consisted of small heterogeneous groups of patients and variable methodology. Nevertheless, the spectrum of altered spindles included “extreme spindles” (high voltage, 200-400µV) recorded in children with intellectual disability due to genetic disorders such as ceroid lipofuscinosis,16 and Costello syndrome,17 lower spindle density in autistic spectrum disorder,18 and increased spindle activity in developmental dyslexia.19 The largest study on the effect of neurological damage on SS was reported by Willis et al.20 It consisted of 180 children aged 2 months to 10 years in whom EEG showed various degrees of SS suppression with ipsilateral of bilateral location. The children had either brain radiological abnormalities or abnormal neurological examination. Unfortunately, the authors did not provide the nature of the etiology of the neurological or radiological abnormalities in their patients. Quite recently Spenner et al.,21 have reported permanent lack of SS development in a significant portion of children with West syndrome. They have also found that delayed appearance of spindles in a subgroup of children with cryptogenic West syndrome could predict a better outcome. Gruber & Weis22 in their comprehensive review of SS characteristic in childhood neurodevelopmental disorders, mainly insomnia, suggested that abnormal SS may contribute to the frequent sleep disorders in this particular population. It is not surprising that we could not find published data on SS in pediatric stroke since awake and sleep EEG is not done routinely during the acute phase of stroke in children if seizures are not suspected. Normal nREM sleep is an important factor in sleep quality as well as general health, however the role of abnormal spindles in sleep impairment following stroke in adults has not been evaluated. Since the long-term outcome of stroke is similar in adults and children,23 we hope that this report will encourage pediatricians and pediatric neurologists to perform sleep EEG in their young patients with stroke and evaluate the long-term effect of abnormal spindles on their sleep quality and daytime alertness.
The effect of hemispheric stroke on spindles in both infants and adults is similar. It is conceivable that such changes may affect the quality of non-REM sleep and as a result impair daytime alertness already documented in adults with stroke and children with a variety of neurodevelopmental disorders.
None.
The authors declare that there are no conflicts of interest.
None.

©2020 Gadoth, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.