eISSN: 2377-4304


Research Article Volume 14 Issue 4
1Clínica de PRONATAL (Hospital Bité Médica), Prolongación Paseo de la Reforma 19, Santa Fe, Paseo de las Lomas, Cuajimalpa de Morelos, 01330 Ciudad de México
2Clínica de Salud Femenina, Insurgentes Sur 03810 Ciudad de México, México
3Horizontes clínica de fertilidad, Calle Josefa Ortiz de Domínguez 538, México
4Clínica FertiFetal, Salud femenina/Reproductiva/Prenatal, Mayorazgo #130, alcaldía Benito Juárez, Hospital San Angel Inn Universidad, México
Correspondence: Lujan-Irastorza Jesús Estuardo, Clínica de PRONATAL (Hospital Bité Médica), Prolongación Paseo de la Reforma 19, Santa Fe, Paseo de las Lomas, Cuajimalpa de Morelos, 01330 Ciudad de México
Received: July 03, 2023 | Published: July 24, 2023
Citation: Estuardo LIJ, Carlos DM, Giovani PPJ, et al. Relationship of morphology and chromatin integrity of sperm in aneuploid blastocyst development: embryos fertilized with sperm diagnosed with teratozoospermia. Obstet Gynecol Int J. 2023;14(4):110-115. DOI: 10.15406/ogij.2023.14.00706
Objective: Evaluate whether the presence of aneuploid blastocysts is associated with sperm morphology and fragmentation.
Methods: Retrospective, observational and cross-sectional study, which included 352 embryos in blastocyst stage, obtained by intracytoplasmic sperm injection (ICSI) from 131 cycles of patients with implantation failure and who decided to perform preimplantation genetic study of aneuploidy (PGT-A) to the embryos that were transferred to the uterus, in order to improve the implantation rate.
Results: Of the embryos obtained from donated oocytes, only those fertilized with semen diagnosed with teratozoospermia presented aneuploidy (26.6%). The rate of aneuploid embryos was similar when own oocytes were fertilized with semen diagnosed with normozoospermia or teratozoospermia (38.4 vs 37.07%). Finally, no relationship was observed between chromatin damage and sperm morphology.
Conclusion: In patients who fertilize their oocytes with spermatozoa from samples diagnosed with normozoospermia or teratozoospermia, the rate of aneuploid blastocysts will depend mainly on the female factor, this does not rule out the possibility of aneuploid embryos due to the male factor. Different from what was seen in donated oocytes, where embryos obtained from semen diagnosed with teratozoospermia presented a higher rate of aneuploidy.
Keywords: recurrent implantation failure; recurrent pregnancy loss, sperm morphology, sperm chromatin, PGT-A
In recent years there has been an increase in female infertility worldwide; in Mexico, rates of up to 17.5% have been reported, which coincides with various international studies.1,2 In addition, it is estimated that 1.5 million couples in the world have been infertile for more than 12 months without using any contraceptive method. This represents 15-20% of couples who have approached an assisted reproduction clinic3 due to difficulty in conceiving.
In addition, chromosomal abnormalities originating in cell duplication by meiosis or mitosis are one of the main causes of infertility, as they have been associated with embryo implantation failure as well as gestational loss. Due to the increase in maternal age. That is to say, advanced maternal age (AMA) the risk of having aneuploid embryos, high risk of miscarriages, implantation failure in in vitro fertilization (IVF) cycles as well as in intracytoplasmic sperm injection, fetal malformations and even the birth of babies with chromosomal disorders increases.3
Interestingly, female infertility and the presence of aneuploidy have also been associated with energy deficiency, since every process that takes place in the human body needs energy to be carried out.4,5 In addition, studies have associated this decrease in energy with alterations in oocyte mitochondria, which may result in inadequate embryo development, implantation failure and gestational loss.6
In view of this situation, assisted reproduction centers have developed technology to evaluate embryos before their transfer to the uterus, which makes it possible to rule out those that present genetic or chromosomal alterations, such as the presence of aneuploidies (monosomies and trisomies in any of the 23 pairs of chromosomes), an example of which is the preimplantation genetic test (PGT).7
PGT is a test developed to analyze DNA from the polar bodies of oocytes or embryos at the third day (cleavage stage) or fifth day of development (blastocyst). PGT was introduced more than 30 years ago as a form of prenatal genetic diagnosis to prevent the transmission of genetic disorders in patients with monogenic diseases (PGT-M), the transmission of chromosomal rearrangements (duplications, deletions, inversions and translocations) (PGT-SR) and to prevent the transfer of aneuploid embryos with monosomies or trisomies (PGT-A), which are one of the causes of implantation failure and gestational loss.8
In order to identify aneuploid embryos, blastocysts are currently biopsied, taking five to ten cells from the trophectoderm, with the advantage of giving more accurate results in PGT, because a larger sample is obtained since they are larger embryos, which generates less impact on viability, compared to biopsies in embryos from the third day (cleavage stage).8,9
Since the implementation of PGT, a large number of technologies have been used for genetic evaluation, such as fluorescence in situ hybridization (FISH), comprehensive chromosome screening (CCS) technology, including real-time quantitative PCR (qPCR), array comparative genomic hybridization (aCGH) and next-generation sequencing (NGS). NGS is the technology currently employed and is of great importance for its high sensitivity in the identification of aneuploidies, while it is characterized by: high throughput, low cost, high sensitivity and specificity; in addition, it can also be used for the identification of translocations and certain point mutations.8,10.
The objective of this study is to evaluate whether the presence of aneuploid blastocysts is associated with sperm morphology and sperm chromatin integrity.
Retrospective, observational and cross-sectional study, which included 352 embryos in blastocyst stage, obtained by intracytoplasmic sperm injection (ICSI) from 131 cycles of patients with implantation failure who attended the PRONATAL clinic located inside the Hospital Bité Médica in Mexico City, in the year 2021; and who in the present study decided to perform preimplantation genetic study of aneuploidy (PGT-A) to the embryos that were transferred to the uterus, in order to improve the implantation rate.
For this study, four groups were formed:
OWN-N: Blastocysts obtained from oocytes aspirated from the same patient who will undergo embryo transfer and fertilized by ICSI with sperm diagnosed with normozoospermia.
PROPIOS-T: Blastocysts obtained from oocytes aspirated from the same patient who will undergo embryo transfer and fertilized by ICSI with sperm diagnosed with teratozoospermia.
OVODON-N: Blastocysts obtained from donated oocytes and fertilized with sperm diagnosed with normozoospermia from the couple that underwent embryo transfer.
OVODON-T: Blastocysts obtained from donated oocytes and fertilized with semen diagnosed with teratozoospermia of the couple that performed the embryo transfer.
The anthropometric data and the procedures performed in the assisted reproduction laboratory were obtained from the patient's history, which was filled out by the nursing staff, as well as embryologists and physicians during the first consultation.
Evaluation of sperm quality (spermatobioscopy)
All sperm samples evaluated were obtained by masturbation in a dedicated area conditioned for masturbation, with an abstinence period of three to seven days. Macroscopic parameters such as volume, appearance (color), liquefaction, viscosity and pH were evaluated, as well as other microscopic parameters such as: concentration, motility (progressive, in situ and immotile), morphology and fragmentation index.
Both sperm morphology and chromatin integrity were assessed using Diff-Quik (Dade Behring Inc., Newark, NJ, USA), composed of methanol (fixative), eosin (dye that stains basic proteins red) and a thiazine-type stain (that stains DNA blue).
Ten µl of the semen sample was placed on a slide and allowed to air dry. The slides were then dipped sequentially for 10 to 20 seconds in each kit solution and then quickly dipped in water to remove excess dye. The slides were allowed to air dry and observed under a brightfield microscope. Chromatin integrity was analyzed using the following staining categories: spermatozoa with light heads/nuclei (normal staining) and spermatozoa with dark heads/nuclei (abnormal staining). To determine the percentage of dark sperm nuclei, 200 cells per sample were counted in four different fields.11 Sperm morphology was evaluated using 2015 WHO criteria.
Controlled ovarian stimulation (COS) and embryo morphology
Controlled ovarian stimulation was performed with the use of a gonadotropin-releasing hormone (GnRH) antagonist on the basis of a short protocol.
The protocol was with administration on day 1 or 2 of gonadotropins (Merional, Merapur or Pergoveys) for 9 days, beginning the application of the antagonist on day 6 or 7 (Cetrotide), until the presence of at least 3 follicles larger than 17mm was observed. Moment in which hCG was applied (day 11 or 12) (Ovidrel). Oocyte retrieval was performed 34 to 36 hours later (follicular aspiration).
Intracytoplasmic sperm injection was used in all procedures. Thus, all embryos were "cultured" until the blastocyst stage was reached. According to Gardner's criteria,12 the morphological scoring of the blastocyst was based on three components: blastocyst expansion, inner cell mass and trophectoderm development.
Preimplantation genetic study of aneuploidy (PGT-A)
A trophectoderm biopsy was performed on blastocysts on the fifth day and sent to a specialized laboratory for PGT-A (Igenomix), which is carried out using the massive sequencing technology (NGS). In addition, for library preparation, the Ion ReproSeqΡTM PGS kit is used in order to perform 24-chromosome aneuploidy analysis together with the Ion ChefΡTM System (Thermo Fisher Scientific, USA). Sequencing of the libraries was performed with the Ion S5 System sequencer (Thermo Fisher Scientific, USA). For data analysis, the Ion Reporter software is used, which performs the alignment of the reads with respect to the latest version of the human reference genome (hg19) (Thermo Fisher Scientific, USA).13 The biopsied blastocysts were vitrified, awaiting PGT-A results for euploid embryo transfer.
Inclusion criteria
Couples with:
Exclusion criteria
Statistical analysis
Parametric data such as anthropometric history and spermogram results are reported as mean ± standard deviation (SD) and the statistical difference was evaluated with Student's t-test. Non-parametric data, such as complications that occurred during pregnancy, aneuploid blastocysts and aneuploidies were reported as percentages and the statistical difference was analyzed with Chi2 test. In both cases the SPSS statistical package was used in its version 25, it was significant p≤0.05.
This study included 352 embryos obtained from 131 cycles that were separated into 4 groups. It was observed that in the anthropometric data DONATION-T presented a statistical increase in age (45.3±2 vs 37.6±4, p≤0.05), weight (70.6±19.8 vs 62.02±5.3, ≤0.05) and height (1.70±0.1 vs 1.56±0.03, p≤0.05) when compared to DONATION-N. As for BMI it was statistically higher in DONATION-N compared to DONATION-T (25.3±3.02 VS 23.9±5.2, p≤0.05). in the case of OWN-T this showed lower in weight (60.1±8.9 vs 63.3±9.8) and BMI (22.9±2.6 vs 24.02±3.2, p≤0.05) compared to OWN-N (Table 1).
|
|
OWN-N |
OWN-T |
DONATION-N |
DONATION-T |
|
Patients (N) |
33 |
69 |
8 |
21 |
|
Blastocysts with PGT-A (N) |
84 |
198 |
20 |
52 |
|
Age (median±SD) |
37.6±4 |
36.7±3.3 |
37.2±3.7 |
45.3±2** |
|
Weight (median±SD) |
63.3±9.8 |
60.1±8.9* |
62.02±5.3 |
70.6±19.8** |
|
Height (median±SD) |
1.62±0.05 |
1.61±0.05 |
1.56±0.03 |
1.70±0.1** |
|
BMI (median±SD) |
24.02±3.2 |
22.9±2.6* |
25.3±3.02 |
23.9±5.2** |
Table 1 Anthropometric data of the mother
*Statistical difference of OWN-N with OWN-N and **Statistical difference of DONATION-N with DONATION-T Student's t-test (p≤0.05).
In the case of the patients included in the study, OWN-T showed a statistical decrease in RIF (10.1 vs 19.04 %, p≤0.05) and a statistical increase in IF (89.8 vs 80.9%, p≤0.05) when compared to OWN-N. In parallel, DONATION-T showed a numerical increase in RIF (11.5 vs 0%) and a numerical decrease in IF (100 vs 88.4%), compared to DONATION-N (Table 2).
|
|
OWN-N |
OWN-T |
DONATION-N |
DONATION-T |
|
Blastocysts with PGT-A (N) |
84 |
198 |
20 |
52 |
|
Female factor |
||||
|
AMA, % (n/N) |
66.6 (56/84) |
63.6 (126/198) |
60 (12/20) |
53.8 (28/52) |
|
RIF, % (n/N) |
19.04 (16/84) |
10.1 (20/198)* |
0 |
11.5 (6/52) |
|
IF, % (n/N) |
80.9 (68/84) |
89.8 (178/198)* |
100 (20/20) |
88.4 (46/52) |
|
Male factor |
||||
|
Volume |
2.5±1.8 |
2.2±1.7 |
2.1±1 |
1.5±0.5** |
|
Concentration |
79.3±44.6 |
61.1±28* |
128.8±38.9 |
51.5±12.2** |
|
Sperm (PM) |
50.2±16.5 |
43.7±19.7* |
63.1±5.8 |
44.3±11.1** |
|
Sperm (NPM) |
14.7±4.7 |
16.4±8.8* |
14.6±2.1 |
17.4±7.9** |
|
Sperm immobile |
35±12.4 |
38.6±18.7* |
22.2±7.5 |
38.2±10.4** |
|
Morphology |
4 |
2.1±0.5* |
4 |
2.7±0.9** |
|
Integridad de la cromatina |
12.5±3.4 |
13.2±2.6* |
13.1±3.5 |
12.8±1** |
|
Ciclo de FIV |
||||
|
MII |
12.2±5.7 |
13.8±4.9 |
10.7 |
11.4±4.9 |
|
Fecundation |
9.8±3.7 |
10.9±4.2 |
8.6±2.9 |
9.3±4.3 |
|
Blastocyst |
3.6±1.2 |
4.2±2.1* |
2.3±0.8 |
4.3±1.7** |
|
FET |
30.9 (26/84) |
30.3 (60/198) |
50 (10/20) |
30.7 (16/52) |
|
Embryo by transfer |
1.05±0.2 |
1.17±0.4 |
1 |
1 |
|
Implantation rate |
69.2 (18/26) |
46.6 (28/60) |
80 (8/10) |
50 (8/16) |
|
Clinical pregnancy |
61.5 (16/26) |
46.6 (28/60) |
80 (8/10) |
50 (8/16) |
Table 2 Euploid blastocyst (PGT-A), female and male factor; and success rate
AMA, advanced maternal age; IF, implantation failure; RIF, recurrent implantation failure; Sperm (PM), sperm with progressive motility and sperm; NPM, sperm with non-progressive motility; MII, metaphase II oocyte; FET, frozen embryo transfer.
*Statistical difference of OWN-T with OWN-N, **Statistical difference of DONATION-T with DONATION-N. Student's t-test (parametric date) and Chi2 test (nonparametric date) (p≤0.05).
As for spermogram results: OWN-T showed statistical decrease of Volume (61.1±28 VS 79.3±44.6, p≤0.05), sperm (PM) (43.7±19.7 vs 50. 2±16.5, p≤0.05) and morphology (2.1±0.5 vs 4, p≤0.05); and statistically increased DNA fragmentation (13.2±2.6 vs 12.5±3.4, p≤0.05) compared to OWN-N. In turn, DONATION-T presented a statistical decrease in volume (1.5±0.5 vs 2.1±1, p≤0.05), concentration (51.5±12.2 vs 128.8±38.9, p≤0.05), sperm (PM) (44. 3±11.1 vs 63.1±5.8, p≤0.05), morphology (2.7±0.9 vs 4, p≤0.05) and DNA fragmentation (12.8±1 vs 13.1±3.5, p≤0.05), compared to Donation-N (Table 2).
In the IVF cycle, OWN-T and DONATION-T reported higher statistical prevalence of blastocysts, compared to their counterparts OWN-N (4.2±2.1 vs 3.6±1.2, p≤0.05) and DONATION-N (4.3±1.7 vs 2.3±0.8, p≤0.05). Of these embryos, the group with the highest FET rate was DONATION-T (50%), compared to OWN-N (30.9%), OWN-T (30.3%) and DONATION-N (30.7%). Implantation rate was numerically higher in OWN-N and DONATION-N, compared to OWN-T (61.5 vs. 46.6%) and DONATION-T (80 vs. 80%). Similarly OWN-N and DONATION-N, showed numerically increased clinical pregnancy rate, when compared to OWN-T (61.5 vs 46.6%) and DONATION-T (80 vs 80%) (Table 2).
In parallel, no significant difference was found in the rate of aneuploid embryos between OWN-N and ONW-T (38.4 and 37.07%), different from what was observed in DONATION-T, which presented a higher rate of aneuploidy compared to DONATION-N (0 vs 26.6%) (Graph 1).
In addition, when the type of aneuploidy was analyzed, it was found that DONATION-N did not present monosomies and DONATION-T only presented monosomies in chromosome 9 (3.3%), 10 (3.3%) and 14 (3.3%). The case of OWN-N was different since it showed a higher number of chromosomes affected by monosomies [2 (2.3%), 9 (2.3%), 11 (2.3%, 15 (4.7%), 16 (2.3%), 18 (2.3%), 15 (4.7%), 16 (2.3%), 18 (2.3%) and 14 (3.3%). 3%), 18 (2.3%), 21 (4.7%) and 22 (4.7%)], similar to OWN-T [4 (2.02%), 7 (2.02%), 8 (2.02%), 11 (1.01%), 15 (1.01%), 16 (3.03%), 18 (2.02%), 19 (1.01%) and 22 (5.05%) (Graph 2).
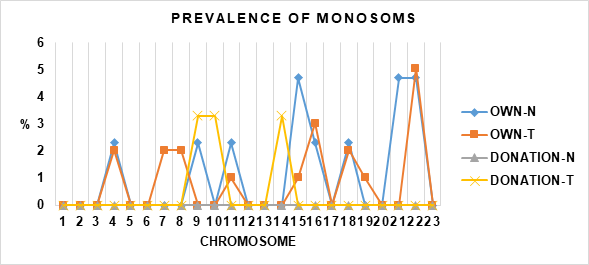
Graph 2 Shows the prevalence of monosomies in each of the 23 chromosomes. Evaluation performed in OWN-N, OWN-T, DONATION-N and DONATION-T.
It was also found that DONATION-N did not present trisomies in any chromosome, compared to DONATION-T who had trisomy in chromosome 1 (3.3%), 5 (3.3%), 6 (3.3%), 12 (3.3%), 16 (3.3%), 20 (3.3%) and 21 (3.3%). Finally, both ONW-N [chromosome 4 (2.3%), 9 (2.3%), 11 (2.3%), 15 (4.7%), 16 (2.3%), 18 (2.3%), 21 (4.7%) and 22 (4.7%)] and OWN-T [4 (2. 02%), 7 (2.02%), 8 (2.02%), 11 (1.01%), 15 (1.01%), 16 (3.03%), 18 (2.02%), 19 (1.01%) and 22 (5.05%)], had trisomies on various chromosomes (Graph 3).
Advanced maternal age plays a crucial role in embryo quality and has long been known to be related to the increase of aneuploid embryos in IVF cycles.14 In this study, it was found that the mean of the four groups exceeded 35 years of age and DONATION-T, presented the highest age (45.3±2 vs 37.6±4, 36.7±3.3 and 37.2±3.7, p≤0.05), weight (70.6±19.8 vs 63. 3±9.8, 60.1±8.9 and 62.02±5.3, p≤0.05) and height (1.70±0.1), a relationship that did not generate overweight unlike DONATION-T, who resulted with higher BMI (25.3±3.02 vs 24.02±3.2, 22.9±2.6 and 23.9±5.2). This may be one of the reasons why these patients have infertility problems, since overweight and obesity are related to the decline in reproductive outcomes in IVF cycles with a decrease in clinical pregnancies and live births and, on the contrary, an increase in spontaneous abortions, without being related to the increase in aneuploid embryos.15,16
Regarding the male factor, we observed a decrease in seminal parameters such as sperm concentration (PM) in the case of OWN-T when compared to OWN-N. As well as the decrease in volume, concentration and sperm (PM) in the case of DONATION-T when compared to DONATION-N. In addition, the samples are still considered normal only in these parameters, according to the criteria of the World Health Organization (WHO) according to the latest revision of the laboratory manual for the review and processing of human semen, published in 2021.17 Likewise, to date there are no studies showing that the numbers obtained in these parameters are associated with increased aneuploidy.18–20
On the other hand, this study shows that OWN-N and OWN-T have similar prevalence of aneuploidies (38.4 vs 37.07%) (Graph 1), as well as they coincide in the presence of monosomies in chromosome 4 (2.02 vs 2.02%), 11 (2.02 vs 1.01%), 15 (1.01 vs 4. 7%), 16 (2.3 vs 3.03%), 18 (2.02 vs 2.02%) and 22 (4.7 vs 5.05%) and with monosomies other than OWN-N on chromosome 9 (2.3%), 11 (2.3%) and 21 (4.7%) compared to OWN-T which showed monosomies on chromosome 6 (2.2%), 8 (2.2%) and 19 (1.01%) (Graph 2).
In parallel, OWN-N and OWN-T coincide with the presence of trisomy 2 (2.3 vs 1.01%), 3 (2.3 vs 1.01%), 6 (4.7 vs 1.01), 16 (2.3 vs 3.3%), 18 (2.3 vs 3.3%), 21 (2.3 vs 2.03), 22 (4.7 vs 1. 01); and differ in the presence in OWN-N of trisomy 17 (2.3%) with OWN-T presenting trisomy 1 (2.02%), 5 (1.01%), 11 (1.01%), 13 (1.01%), 14 (4.04%), 15 (1.01%),19 (3.03%), 22 (1.01%) and 23 (3.03%) (Graph 3). This is different from what was reported by Kiseleva Y. et al., 2017, in study that included 46 couples, in which the male presented sperm morphology ≤4% and when compared with control group with morphology >4, it was found that embryos obtained from own eggs and semen diagnosed with teratozoospermia presented higher prevalence of autosomal monosomies (46.2 vs 26.4%), autosomal trisomies (48.5 vs 25.5%) and sex chromosome trisomies (7.2 vs 3.4%).21 Similarly, Rodriguez J. et al., 2016, it was observed that the rate of aneuploid embryos obtained from own eggs and semen with morphology less than 2% was higher, when evaluating almost 8000 embryos.22
In the case of DONATION-N, there were no aneuploidies, in contrast to DONATION-T, which presented a rate of 26.6%. In the latter, monosomy was observed in chromosome 9 (3.3%), 10 (3.3%) and 14 (3.3%) (Graph 2); and trisomy in chromosome 1 (3.3%), 5 (3.3%), 6 (3.3%), 12 (3.3%), 16 (3.3%), 18 (3.3%), 20 (3.3%) and 21 (3.3%) (Graph 3). This could indicate that fertilization of donated eggs with semen diagnosed with teratozoospermia could be associated with increased aneuploid blastocysts, coinciding with Coban O. et al., 2018, who in study that included 1165 ovodonation embryos that were fertilized with semen diagnosed with teratozoospermia (1 to 4, by strict kruger criteria), observed the decrease in morphology presents inversely proportional relationship with the increase of aneuploidies (monosomies and trisomies of chromosome 13, 18, 21 and 23).23
As can be seen, aneuploidies are not only associated with the female factor, but there is also a certain percentage related to the male factor. It is known, as a result of abnormal chromosome segregation, which occurs during meiosis and can lead to aneuploidy in both female and male gametes by removing and including an extra copy of some chromosome, and the most frequent is aneuploidy in spermatozoa of approximately 4.5% and oocytes in approximately 20%.23
There was an increase in fragmentation index with respect to chromatin integrity or sperm DNA fragmentation (SDF) index in OWN-T and DONATION-N compared to their counterpart OWN-N (13.2±2.6 vs 12.5±3.4, P≤0.05) and DONATION-T (13.1±3.5 vs 12.8±1, p≤0.05). Thus, all four groups showed sperm fragmentation index <15% which is classified with good fertilization potential,24,25 preventing to observe relationship of SDF and development of aneuploid embryos. For its part, SDF can be caused by extrinsic factors such as: exposure to heat, smoking, environmental pollution and chemotherapy; as well as intrinsic factors, such as defects in germ cell maturation, abortive apoptosis and oxidative stress, all resulting in male infertility, as well as decreased fertilization rate and embryo development.26–28 Furthermore, a large number of studies agree that there is no association of SDF with the development of aneuploid embryos.27–29
On the other hand, in OWN-T (4.2±2.1) and DONATION-T (4.3±1.7) a higher blastocyst rate was obtained in comparison with OWN-N (3.6±1.2) and DONATION-T (2.3±0.8), coinciding with previous studies, where the selection of spermatozoa with normal morphology by microscopy during ICSI, allows excellent results even with samples with severe teratozoospermia.30,31 In addition, we confirmed that the embryonic development of OWN-T and DONATION-T was not affected by the fragmentation index because it was low in all four groups.
Finally, the implantation and clinical pregnancy rate was lower in OWN-T (46.6 and 46.6%) and DONATION-T (50 and 50%) compared to OWN-N (69.2 and 61.5%) and DONATION-N (80 and 80%). We still do not know the exact mechanisms by which embryos from semen diagnosed with teratozoospermia presented a lower implantation rate. But there are studies which suggest that spermatozoa selected for ICSI may have undetectable structural alterations at 400X such as nuclear vacuoles, which decreases the pregnancy and implantation rate. Therefore, more sensitive techniques such as intracytoplasmic injection of morphologically selected spermatozoa (IMSI) are recommended, where better implantation rates have been obtained when compared with ICSI.32
When intracytoplasmic sperm injection (ICSI) is performed by selecting normal spermatozoa from samples diagnosed with teratozoospermia, the rate of aneuploid embryos is increased when they are used to fertilize donor oocytes.
In addition, the rate of aneuploid embryos is similar when fertilizing own oocytes with semen diagnosed with normozoospermia or teratozoospermia.
One the other hand, in patients who fertilize their oocytes with spermatozoa from samples diagnosed with normozoospermia or teratozoospermia, the rate of aneuploid blastocysts will depend mainly on the female factor. This does not rule out the possibility of aneuploid embryos due to the male factor.
The prevalence of trisomy 18 (2 to 3%) and 21 (2 to 3%) is similar in blastocysts obtained from own and donor oocytes that were fertilized with semen diagnosed with teratozoospermia. The prevalence does not change in embryos obtained from own oocytes fertilized with sperm diagnosed with normozoospermia.
Likewise, teratozoospermia in our study did not increase the risk of embryos with trisomy 13. In fact, the implantation rate is lower in embryos from own or donated oocytes fertilized with sperm diagnosed with teratozoospermia.
Finally, a prospective study with a larger number of embryos is needed to determine whether the Mexican population, which is characterized by a high prevalence of teratozoospermia, behaved in the same way as the population in this study (Pronatal Clinic).
None.
None.
The authors declare that they have no conflict of interest.

©2023 Estuardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.