eISSN: 2377-4304


Case Report Volume 7 Issue 3
1Department of Gynaecology and Obstetrics, Pula General Hospital, Croatia
2Department of Urology, Pula General Hospital, Croatia
Correspondence: Dragan Belci Department of Gynaecology and Obstetrics, Pula General Hospital, Pula, Croatia
Received: January 05, 2017 | Published: June 30, 2017
Citation: Belci D, Mihovilić N, Zoričić D, et al. Spontaneous lippes loop IUCD intravesical migration with formation of bladder calculus and vesicovaginal fistula: a case report. Obstet Gynecol Int J. 2017;7(3):261-263. DOI: 10.15406/ogij.2017.07.00250
Intrauterine contraceptive device (IUCD) is one of the most frequently used contraceptive methods worldwide. Spontaneous perforation of the uterus or vagina and IUD migration to the bladder is very rare. We present a case of 68-year-old female with vaginal perforation of IUCD and migration into the bladder which resulted in formation of bladder calculus.
As an invasive method of contraception, IUD has its disadvantages. The most frequent side effects, especially associated with the use of Lippes Loop IUDs, are irregular uterine bleeding, pain in the lower abdomen, unplanned pregnancy, spontaneous abortion and pelvic inflammatory disease (PID). Spontaneous expulsions are rare, but can occur in the first months of usage, especially when the IUD is inserted shortly after childbirth. Uterine perforation is an extremely rare complication with a rate of 0.4/1000 insertions.1 Perforation can occur during the insertion of IUD so it is recommended to be performed by an experienced gynaecologist.2 Due to IUD migration, 30% of uterine perforations are asymptomatic.
A 68-year-old female, gravida 3(1 missed abortion), para 2, postmenopausal for 14 years, who came to the gynaecology outpatient department, presented with pain in suprapubic region for a year. Because of frequent urinary tract infections and mixed urinary incontinence, during the course of a year she took propiverine hydrochloride 30 mcg and nitrofurantoin 50 mcg prescribed by a general practitioner. Her last gynaecological examination was 20 year ago. After the second birth in 1970, sutures were put on her vagina and cervix and she received a blood transfusion. She cited that she was put an IUD 40 years ago. So far she suffered from arterial hypertension, glucose intolerance and GERD.
At this point vaginal examination was done- vagina was closed 2 cm proximal to the vaginal introitus and no cervix was visualised. Vaginal smear was taken for cytological analysis which was negative for intraepithelial neoplasia. There was no clear outline of the uterus and ovaries visible on transrectal ultrasonography. IUD was not visualised and there was no fluid in the Pouch of Douglas. Transabdominal ultrasound showed intravesical hyperechoic reflection 3.4 cm in diameter which could represent stone. Further pelvic and abdominal CT scan revealed hyperdense linear formation with dimensions of 2.9x 5.0 cm placed in the uterine isthmus and vagina that descends to the introitus of the vagina. Furthermore, round calcified formation with dimensions of 3.0x2.4 cm was revealed in the posterior bladder wall (Figure 1). These two formations were in close contact.
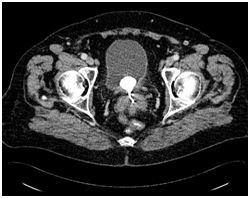
Figure 1 Pelvic computed tomography shows 3.0x 2.4 cm – sized bladder calculus in close contact with the IUD placed in the vaginal fornix.
Laboratory parameters were within normal values. Urine culture demonstrated Proteus mirabilis infection so the patient was given cefuroxime intravenously for 7 days at the Department of Infectious Diseases. When the urine culture came sterile, the patient was examined by aurologist for the first time and cystoscopy was performed. The urological operation was planned based on uroendoscopy and ultrasound findings where the stem (calculus) was verified. IUD was not verified because the stem was completely obliterated by the IUD. The operation was performed endoscopically/transuretral/ without the opening of the bladder. It revealed a stone formation within the base of the bladder which was broken with a mechanical lithotriptor. After the procedure it was observed that the IUD was within the bladder.
The above approach / endoscopy-transurethral / was not possible to remove IUD. Intraoperatively consulted a gynecologist and an additional MSCT diagnost procedure was agreed with the aim of accurate prosthetic position verification and trans operative transplant surgery.
The patient was scheduled for an operation at our Department of Gynaecology. Due to the difficulty of IUD visualisation, total hysterectomy with bilateral salpingo-oophorectomy was done by laparotomy. Uterine surface was intact. During the opening of the vagina, it was visualised that the calcified IUD with its one part broke through the vagina and with the other part it was situated in the vaginal fornix. The IUD was extracted and the vagina was closed using single sutures. No leakage was observed after the bladder was filled with 200 ml methylene blue. The operation was concluded (Figure 2).
Histopathological analysis indicated endometritis, fallopian tubes fibrosis and involutive ovarian changes. Patient was given intravenously cefazolin 2x1.0 g and crystalloid solutions. In early postoperative recovery, the patient presented with dyspnoea. MDCT pulmonary angiography, ECG and laboratory tests were performed to exclude pulmonary embolism. Low molecular weight heparin in dose of 2x 5 000 IU was administered for 17 days. Foley catheter was kept postoperatively for 14 days. Shortly after the removal of the catheter, the patient presented with signs of vulvovaginal infection, frequent urinating without incontinence and suppuration of the postoperative wound. Control cystoscopy revealed a small fistula opening between the mouths of ureters. Urine culture was sterile, while the wound swab was positive for Proteus mirabilis. Foley catheter was administered again. Patient was given intravenously cefuroxime 3x 1.5 g and metronidazole 3x 500 mg for 14 days, which was then replaced with ciprofloxacin 500 mg 2x1 pill per day. The patient was released from the hospital to home care. After 4 weeks the catheter was removed at the urology outpatient department. Control cystoscopy revealed a scar after healing of the fistula and an orderly appearance of the bladder mucosa. Bladder capacity was 350 mL and the stress test was negative
It has been known for centuries that things placed in uterus prevent pregnancy, but Richter was the first one who reported about his experiences with IUDs in 1909. LippesLoop IUD, which is presented here, was first distributed in 1962. It is a plastic double “S” loop of size appropriate for the uterine cavity. The incidence of uterine perforation caused by LippesLoop IUD was approximately 0.5- 8.7/1000 insertions.3 Nowadays, intrauterine contraceptive devices (IUCDs) present a safe, convenient and effective method of contraception when used properly.
In this case we presented a postmenopausal woman with a neglected IUD placed 40 years ago. We cannot determine the exact time of spontaneous IUD expulsion, but complications could follow immediately after it occurs. Bladder perforation with formed bladder calculus, vesicovaginal fistula and subsequent vaginal stenosis were discovered. We performed transrectal sonography to seek an IUD but were unable to identify it. However, the transabdominal sonography was useful in detecting the bladder calculus. X-ray, computed tomography or magnetic resonance should be performed for IUD visualisation and its relation to the surrounding structures.4
Although IUD migration through uterus and bladder perforation is extremely rare, we found cases describing this pathology. Mixed urinary incontinence, dysuria, haematuria, vaginal discharge and chronic lower abdominal pain may be present in case of bladder involvement.2,4-6 However, we found only two cases of vaginal IUD perforations with formed vesicovaginal fistula and vesicovaginal calculus. In one case the patient presented with signs of acute kidney failure and metabolic acidosis. After the initial haemodialysis treatment, vaginal lithotomy was performed to extract parts of an old IUD- LippesLoop. The patient refused secondary cystolithotomy and fistula repair. In other case of migrating SaF- T- Coil the patient underwent cystoscopy and cystolithotripsy of a large intravesical stone. The IUD was removed via vaginal route and vesicovaginal fistula was treated.7,8
There are no clear guidelines on how to perform the IUD removal. The Lippes Loop IUCDs tend to cause calcium precipitation leading to corrosion in the plastic. They are difficult to remove especially after prolonged use in postmenopausal age due to chronic local inflammation and fibrosis.9 Also, there was no doubt that the patient will undergo hysterectomy due to risk factors for developing uterine adenocarcinoma (postmenopausal for 14 years, overweight with BMI 29.4, glucose intolerance). Initially we performed cystoscopy with bladder calculus lithotripsy to visualise and free one part of the IUD. Because of the vaginal stenosis and IUD perforation of the bladder wall we performed laparotomy, aware of all the consequences of open surgery, of which some occur in early and late postoperative recovery.5
Bladder calculus is unusual in female patients and aroused a suspicion of a bladder perforation caused by a foreign body. When the bladder is perforated, urinary infection facilitates stone formation. The patients presented with mixed urinary incontinence and recurrent UTIs which usually respond only temporarily to therapy, and a history of placed IUD must be examined by an experienced gynaecologist.4 When IUD migration is suspected, imaging methods are necessary to determine the exact position of the IUD. Surgical approach is required to treat complications caused by perforation of surrounding organs. When possible, minimally invasive surgery should me a method of choice. If VVF is diagnosed or there is a suspicion of it, we recommend that the Foley catheter be kept postoperatively for 28 days while administering a broad-spectrum antibiotic for that period of time.
None.
None.

©2017 Belci, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.