eISSN: 2377-4304


Research Article Volume 5 Issue 4
Cebu Doctors University College of Medicine, Philippines
Correspondence: Helen Valenzona Madamba, Associate Professor, Cebu Doctors University College of Medicine, Dr. P. V. Larrazabal Jr. Avenue, North Reclamation Area, Mandaue City, Cebu, Philippines
Received: September 19, 2016 | Published: November 7, 2016
Citation: Madamba HV. Disseminated tuberculosis with involvement of the bone marrow. Obstet Gynecol Int J. 2016;5(4):368-374. DOI: 10.15406/ogij.2016.05.00167
Background: Tuberculosis (TB) is still a major public health concern in the Philippines. With nationwide efforts for TB control in place to achieve the sixth millennium development goal, it is important to be familiar the unusual presentations of disseminated tuberculosis.
Case: G.P. is a 31 year old nulligravid with a four‒month history of progressive abdominal enlargement due to ascites, associated with fever, chronic cough, early satiety, anorexia, and significant weight loss. Diagnosed as a case of ovarian malignancy, work up for tuberculosis revealed acid‒fast bacilli isolated in the sputum, peritoneal fluid, cervical discharge and urine. The patient manifested with ecchymosis and petechial rashes over her chest, arms and extremities a day after she was started on fixed drug combination of isoniazid, rifampicin, pyrazinamide and ethambutol. Complete blood count showed anemia and thrombocytopenia. Considering an allergic reaction, the medications were immediately discontinued. However, with no improvement in symptoms, a bone marrow aspirate and core biopsy was done and results were compatible with tuberculous etiology.
Conclusion: Disseminated tuberculosis may present as a diagnostic dilemma considering tuberculosis may mimic ovarian malignancy. It is important to differentiate one from the other as management will differ. It is as important to rule out malignancy when tuberculosis is present, as both may co‒exist. Bone marrow biopsies and blood TB cultures are also useful in diagnosing disseminated tuberculosis especially when diagnosis is difficult. Prompt diagnosis and treatment with quadruple anti‒Koch’s medications may be life‒saving for these complicated cases.
Keywords: Tuberculosis, Anemia, Thrombocytopenia, Bone marrow, Ovarian malignancy
TB, Tuberculosis; NTP, National Tuberculosis Control Program; DOTS, Directly Observed Treatment Short‒Course; DOH, Department of Health; SDF, Single Drug Formulation, FDC, Fixed Dose Combination; MDG, Millennium Development Goals; UN, United Nations; WHO, World Health Organization; NTPS, National Tuberculosis Prevalence Study; NCDPC, National Center for Disease Prevention and Control; Phil PACT, Philippine Plant of Action to Control Tuberculosis; PIPH, Province wide Investment Plan for Health; AIPH, ARMM Investment Plan for Health; AFB, Acid‒Fast Bacilli
Tuberculosis (TB) is still a major public health concern in the Philippines, ranking as the sixth leading cause of morbidity and mortality based on recent local data. Worldwide, there were 9.27 million incident cases of tuberculosis in 2007, of which 4 million were smear positive cases. Asia accounted for 55% of the cases while Africa contributed 31%.1 The Philippines ranked ninth among the 22 high burden countries that accounted for 80% of the TB burden. This is two ranks lower than the seventh place that the country occupied in 1998.2 The national TB prevalence survey carried out in 20073 showed that the prevalence rate of smear positive TB was 2 per 1,000 (200 per 100,000) while culture positive was 4.7 per 1,000 (470 per 100,000). The prevalence of tuberculosis is highest among poor, elderly and urban dwellers.
Health programs for TB control in the philippines
In 1996, the National TB Control Program (NTP) implemented the directly observed treatment short‒course (DOTS) strategy with active participation of the local government units. In 1998, the NTP became one of the flagships of the Department of Health (DOH), and achieved nationwide coverage in the public health sector in 2003, with expansion involving the private sector through the Public‒Private Mix DOTS strategy.4 All public health centers, regional health units and their substations were utilizing the NTP policies and the DOTS strategy for their local TB control efforts. To improve patient compliance, the NTP maintained the quadruple therapy but shifted its policy from use of single drug formulations (SDF) to fixed dose combination (FDC). This is the present treatment formulation under the NTP for all DOTS facilities. In spite of all these achievements, various problems linked to factors attributable to the patient, the health care provider, and the program contributed to the persistence of tuberculosis in the country.
The sixth millennium development goal (MDG) of the United Nations (UN),5 member countries and partner organizations, includes reducing the prevalence of tuberculosis. In 2006, World Health Organization (WHO) projected that the Philippines would meet the MDG targets in 2015. This projection may not be met given the lower annual rate of decline of the TB prevalence of only around 2% as revealed by the 2007 National Tuberculosis Prevalence Survey (NTPS).2 Spurred by its desire for the Philippines to achieve the millennium development goals (MDG) for TB control in 2015, the National Center for Disease Prevention and Control (NCDPC) of the Department of Health (DOH) started the task of reviewing and updating the 2006–2010 National Strategic Plan to control TB in January 2009. The 2010‒2016 Philippine Plan of Action to Contol TB (Phil PACT), defined by multisectoral, broad based collective and technical inputs from various national and local agencies and partners, underlines the key strategic approaches towards achieving these targets at the national level. The NTP is spearheading once again a more comprehensive strategic plan that would incorporate the TB control plans to the Provincewide / ARMM Investment Plan for Health (PIPH/AIPH).
Disseminated tuberculosis
Diagnosis of tuberculosis refers to the recognition of an active TB case, a patient who is symptomatic due to lesions caused by Mycobacterium tuberculosis confirmed by microbiologic studies. Cases are classified as pulmonary tuberculosis or extra‒pulmonary tuberculosis, according to the site affected. Disseminated tuberculosis refers to active hematogenous spread of Mycobacterium tuberculosis in two or more organs/systems in the body leading to a generalized systemic illness.6 Disseminated tuberculosis is also defined as tuberculous infection involving the blood stream, bone marrow, liver or military tuberculosis.6 It can present with various clinical features7‒14 and is a potentially lethal form of TB resulting from massive lymphohematogenous dissemination of M. tuberculosis bacilli.7 Delayed presentation is a factor for high mortality rate,15 hence the importance of timely intervention with anti‒tuberculous treatment.
This paper aims to present a case of disseminated tuberculosis who presented with massive ascites, initially diagnosed as ovarian malignancy, who subsequently developed bicytopenia, suggesting tuberculous infiltration and involvement of the bone marrow (Figure 1).
This is the case of G. P. a 31 year old Roman Catholic, married, nulligravid Filipino from Samar, who was admitted at our institution for the first time due to difficulty of breathing due to progressive abdominal enlargement.
Her past medical history was unremarkable, with no history of hypertension, diabetes mellitus, allergy, bronchial asthma, heart or lung disease, goiter or malignancy. She had no prior history of tuberculosis, nor any history of hospitalization or operations. Her mother had tuberculosis, treated for 6 months with quadruple anti‒Koch’s medications. Her father had hepatitis infection and died of liver cirrhosis. She has no vices, is a housewife, with her first sexual contact at 22 years old to a single non‒promiscuous sexual partner. She has never been pregnant despite nine years of regular sexual intercourse with her husband without using any form of family planning methods. She had her menarche at 14 years old, with subsequent menses occurring at regular monthly intervals, consuming 3 pads per day lasting 4 days with no history of dysmenorrhea. She has been amenorrheic for the past five years.
The patient presented with a 4‒month history of progressive abdominal enlargement causing intermittent abdominal pain and difficulty of breathing with 3‒pillow orthopnea. Patient also experienced undocumented fever, chronic cough, early satiety, anorexia, and weight loss of more than 20% from baseline. She had no urinary nor bowel changes. Persistence of symptoms prompted consult with a private physician in Ormoc, Leyte. Ultrasound showed a solid mass on the left hepatic lobe, with multiple calcifications on the right hepatic lobe. Chest x ray showed interstitial pneumonia. Serum AFP was allegedly normal, while CA 125 (508.2 IU/mL) and CEA (5.75 IU/mL) showed elevated values. The patient was referred to our institution for further evaluation and management.
On admission, the patient was cachectic, conscious, coherent, ambulates with assistance, not in cardio respiratory distress. Vital signs were stable with blood pressure 120/80, heart rate at 80 beats per minute, respiratory rate at 20 cycles per minute. She was a febrile with temperature of 37.4°C. She weighed 41 kg and stood 148 cm. She had pink palpebral conjunctiva, anicteric sclera, no tonsillopharyngeal congestion, no anterior neck mass, no cervical lymphadenopathies. She had equal chest expansion, no wheezes, no crackles, with decreased breath sounds at the basal lung fields, adynamic precordium and distinct heart sounds with no note of murmurs. The abdomen was globularly distended to a girth of 92 cm but was soft, with normoactive bowel sounds and no tenderness. On percussion, there was fluid wave and shifting dullness. There was grade II bipedal pitting edema. Speculum exam showed smooth pink vagina and cervix with note of white caseous material at the cervical os and creamy discharge over vaginal walls. Internal examination revealed normal external genitalia, smooth nulliparous vagina, cervix closed and smooth, corpus seemed small. The adnexae could not be adequately assessed due to massive abdomino‒pelvic fluid. On recto vaginal examination, there was tight sphincteric tone, empty rectal vault, no masses, no tenderness, brown stool per examining finger.
With the four‒month history of rapid abdominal enlargement and significant weight loss, the admitting impression was that of an ovarian new growth probably malignant. However, ultrasound showed only a left adnexal mass described as irregular tubular hyperechoic structure with scattered echogenic stipplings within, densely adherent to the postero‒lateral wall of the uterus, adjacent bowel loops and pelvic sidewalls, while the right ovary appeared grossly normal. Incidentally, there were multiple echogenic foci seen at inferior lobe and parenchyma of the liver, and massive anechoic free fluid was seen in the abdominopelvic cavity. A chest radiograph was also done, which showed pleural effusion on the right; findings suggestive of pulmonary tuberculosis, minimal disease. Work up for tuberculosis was done (Figures 2‒10).
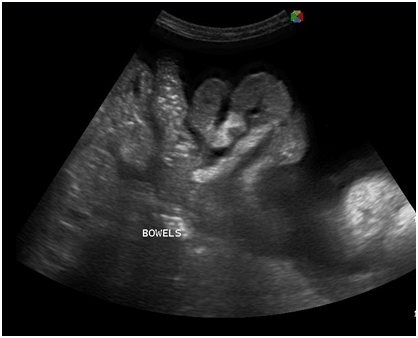
Figure 5 Transabdominal view showing massive anechoic free fluid in the abdominopelvic cavity (ascites) and pallisading bowels.

Figure 7 There is a thin midline echogenicity visualized (anteroposterior view), measuring 0.3 cm suggestive of the endometrial lining.
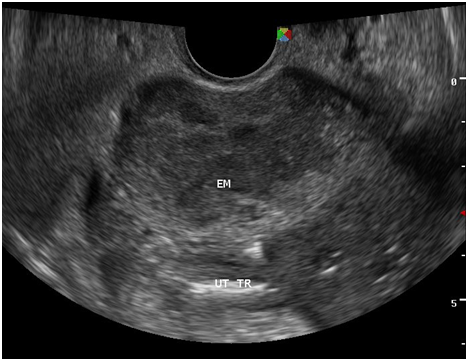
Figure 8 The surrounding myometrium (transverse view) which is hypoechoic, irregular, with scalloping at the periphery with a mixed hyperechoic and echogenic echopattern giving it a marblelized appearance, which may be a unique variation of the usual “moth‒eaten” appearance.
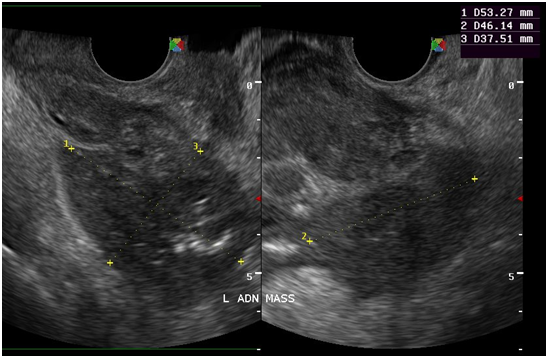
Figure 10 At the left adnexal area is an irregular tubular hyperechoic structure with scattered echogenic stipplings within.
On the 3rd hospital day, the patient underwent ultrasound‒guided paracentesis, and 4.8 liters of serous fluid was drained which showed (+)1 acid‒fast bacilli (AFB). By the 5th hospital day, results for tuberculosis work up showed (+)2 AFB in sputum, (+)1 AFB in cervical discharge, and (+)5 AFB in urine. The patient was referred to and transferred to obstetric and gynecologic infectious disease service. The working impression at this time was disseminated tuberculosis (pulmonary, genitourinary, peritoneal, gastrointestinal); massive ascites and pleural effusion secondary to tuberculosis, status post ultrasound‒guided paracentesis; secondary amenorrhea or five years due to abdomino‒pelvic Koch’s infection; primary infertility for nine years.
Quadruple anti‒tuberculosis medications (Isoniazid, Rifampicin, Pyrazinamide, Ethambutol [HRZE]) were started as fixed combination of 3 tablets once a day 30 minutes pre‒breakfast with vitamin B complex 1 tab once a day. After two days on this medication regimen, multiple bruises over the arms and petechial rashes over the abdomen were noted. An adverse drug reaction to anti‒tuberculosis medications was considered, especially to rifampicin, which is notorious for causing hematologic derangements. The medications were immediately discontinued. Complete blood count and bleeding parameters were requested. The patient was noted to have thrombocytopenia with platelet count of 5 x 109/L. Platelet concentrates were transfused at 4 units bolus every 12 hours. The patient was referred to Allergy Service. The impression was thrombocytopenia secondary to adverse drug reaction to rifampicin. The plan was to correct the hematologic derangement and reintroduce the anti‒tuberculosis medications as individual tablets, no longer as fixed combination doses.
On the 10th hospital day, the patient’s blood picture showed not only thrombocytopenia but anemia as well, with a drop in the hemoglobin level from 91 to 78 mg/dL. The patient was referred to hematology to rule out a primary blood dyscrasia. Working impression was bicytopenia, multi factorial, secondary to chronic inflammation, rule out tuberculous infiltration of the marrow. They agreed with restarting anti‒tuberculosis meds as soon as possible to address overwhelming infection. Fixed dose combination quadruple anti‒tuberculosis medications were started and tolerated well.
To relieve difficulty of breathing due to massive ascites, a second ultrasound‒guided paracentesis was done, and 2.2 liters of serosanguinous ascitic fluid was drained. Bone marrow aspiration was done, and samples were sent for TB culture and histopathology. The patient tolerated the procedure well. Blood transfusion with 3 units packed red blood cells raised hemoglobin to 128 mg/dL. Four units platelet concentrate was continued every 12 hours to prevent active bleeding. The latest platelet count was 6 x 109/L. Unfortunately, due to lack of financial and logistic support, the patient opted to go home against medical advice. She was referred to the nearest health center for directly observed therapy for disseminated tuberculosis. Although adequately advised regarding follow‒up, the patient never came back even at the outpatient clinic. She however kept in touch through phone calls. As of writing, the patient is living in Ormoc City, Leyte, clinically improved with a flat abdomen and weight gain and as is currently on the 7th month of her treatment regimen at TB DOTS.
Tuberculous peritonitis versus ovarian cancer
The patient presented with massive ascites and was initially diagnosed as a case of ovarian new growth. Peritoneal tuberculosis poses a major diagnostic challenge with the lack of specific clinical and laboratory markers.16 Tuberculosis is the great mimic – patients with TB peritonitis present with non‒specific signs that may be misdiagnosed as ovarian cancer17,18 such as abdominal pain, fever, weight loss, constipation, ascites, hepatomegaly and splenomegaly.19,20 A high clinical index of suspicion and awareness of tuberculous peritonitis in susceptible individuals is mandatory. When left untreated, tuberculosis peritonitis can be fatal but can be cured if diagnosed promptly and appropriate treatment is started.16 Ascitic fluid smear sent for acid‒fast bacilli microscopy or polymerase chain reaction19 for M. tuberculosis and endometrial biopsy may help to distinguish pelvic‒peritoneal tuberculosis from ovarian malignancy. Peritoneal tuberculosis can be managed through chemotherapy with anti‒tuberculosis medications, therefore these test should be performed before surgery to exclude peritoneal tuberculosis, so that invasive and expensive surgery could be avoided,18,21 as was done in this patient.
Tuberculous peritonitis is a variant of genital tract tuberculosis. Fallopian tube/uterine disease and tuberculous peritonitis can and does coexist in up to 50% of the cases.22 Tuberculosis is primarily an infection of the respiratory tract. Female genital tuberculosis can develop from a pulmonary nidus of infection or by hematogenous dissemination of organisms and their subsequent localization within the fallopian tube. When the gastrointestinal tract is the portal of infection, involvement of the ileocecal region permits lymphatic spread–primarily to the right fallopian tube.22 The sono graphic findings of this patient described a 5.3x4.6x3.8 cm tubular adnexal mass with scattered echogenic stipplings within, which probably represented tuberculous salpingitis. Myometrial involvement in this patient appeared as irregular scalloping at the periphery of the surrounding hypoechoic myometrium, giving it a marblelized appearance. Dissemination of disease from the fallopian tube occurs by continuous spread to potentially involve the ovary and retrograde into the uterus. Uterine extension involves primarily the endometrium with, at maximum, a 20% incidence of myometrial involvement.22
Disseminated tuberculosis
Disseminated tuberculosis is uncommon. In a local study,6 (2001) identified 13 cases of disseminated tuberculosis which accounted for only 0.6% of all new cases of tuberculosis (2,030 patients) over a ten‒year period from January 1987 to February 1997. The mean age was 29 years old with a M: F ratio of 1:1.6. The mean duration of illness prior to diagnosis was 9.8 months. The most common presenting symptoms were fever (69.2%), night sweats (46.1%), abdominal pain (46%), cough (38.5%) and weight loss (38.5%). Eleven (84.6%) patients were believed to have concomitant active pulmonary tuberculosis on basis of clinical manifestations, pulmonary infiltrates on chest radiographs and sputum bacteriologic testing.
Hakawi & Alrajhi et al.15 did a clinico‒pathological study on 22 cases of tuberculosis of the bone marrow from 1990 to 2002. They identified 22 patients with culture‒proven Mycobacterium tuberculosis from bone marrow specimens. The median age of patients was 48 years old and the median time to presentation (calculated from time from start of symptoms to presentation to a health care facility) was 3 months. The presenting complaint was fever and weight loss for all 22 patients. Only 5 patients (23%) were reported to have normal complete blood count. Anemia (defined as hemoglobin <10 g/dL) was seen in 20 patients (91%), leucopenia (defined as white blood cell count <3x109/L) in 17 patients (77%), thrombocytopenia (defined as platelet count <100x109/L) in 10 patients (45%) and lymphopenia (defined as lymphocyte coung <1.5x109/L) in 17 patients (77%). Histopathological findings were reported as granuloma in 19 patients (86%), whereas only 3 patients (14%) were reported to have caseating granulomas. The outcome was favorable for 11 patients while 10 patients died during hospitalization and one was lost to follow‒up. The high mortality rate was attributed to delayed presentation. Most patients with bone marrow TB died a mean of 2 weeks after initiating anti‒tuberculosis treatment. For patients who completed their regimens, it is interesting to note that the duration of treatment was 12 months despite current recommendations that a six‒month treatment is adequate for treating patients with disseminated TB excluding CNS involvement. They conclude that isolation of Mycobacterium tuberculosis from a bone marrow specimen is an indication of disseminated disease which carries a high mortality rate and requires prompt initiation of appropriate treatment.
In Taiwan, Wang et al.7 studied 164 out of 3058 culture‒confirmed TB patients (5.4% prevalence) who fulfilled the criteria for disseminated tuberculosis from January 1995 to December 2004. 87 patients (53%) had underlying co‒morbid conditions such as AIDS, diabetes mellitus, malignancy, end‒stage renal disease, autoimmune disease, and alcoholism. Within one year of follow‒up, 99 (60.4%) patients were completely treated and 51 (31%) died, 10 (6.1%) were under treatment and 4 (2.4%) were lost. Poor prognosis factors reflect the severity of TB or the underlying diseases, and include albumin <3.5 g/dL, total bilirubin >1.0 mg/dL, creatinine >1.5 mg/dL and delayed anti‒tuberculosis treatment. This patient had positive AFB smears from all over her body (lungs, urine, stool, pelvis, bone marrow) – suggesting an immuno compromised state such as HIV/AIDS or a person with malignancy or diabetes mellitus. However, the result of HIV screening for this patient was nonreactive and her blood sugar levels were with normal limits. Patients with disseminated tuberculosis have varied presentations which may be lethal if detection is delayed, including pancytopenia,8 hemophagocytic syndrome,9,11 bone marrow necrosis,13 disseminated intravascular coagulation,10 isolated thrombocytopenia.14 Disseminated tuberculosis is treated with quadruple anti‒Koch’s medications, under the DOTS strategy. Interestingly, although the current recommendation is a six‒month treatment regimen for disseminated TB, patients with disseminated tuberculosis are treated for a minimum of 12 months in Saudi Arabia.15 The same is true for our section. However, anti‒tuberculosis medications do have its inherent risks and complications. Petechial rash may suggest thrombocytopenia in patients taking rifampicin.4 The platelet count was checked immediately in our patient and since it was initially low, rifampicin hypersensitivity was identified as the cause. The medications were stopped and hematologic disturbances were corrected prior to resuming the medications. The plan was to restart the medications one by one at intervals of 2‒3 days once the rash improved. However, since there was no observed improvement despite cessation of the medications, a primary hematologic disorder was considered (Figures 11‒14).
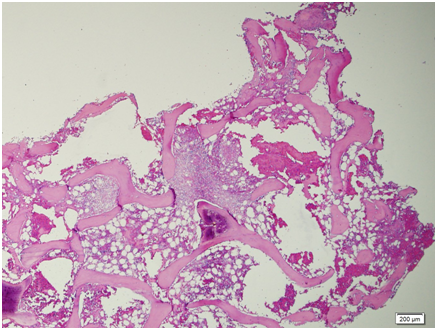
Figure 12 Scanning view: the bone marrow aspirate smear is cellular and polymorphous showing myeloid and mild increased in erythroid precursors in all stages of maturation with an M/S ratio of 1.2:1. No extraneous cells noted. Megakaryocytes are identified.
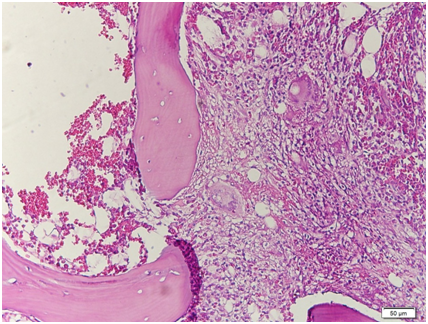
Figure 13 Low power view. The bone marrow core biopsy is hypercellular for age showing 80‒90% cellularity.
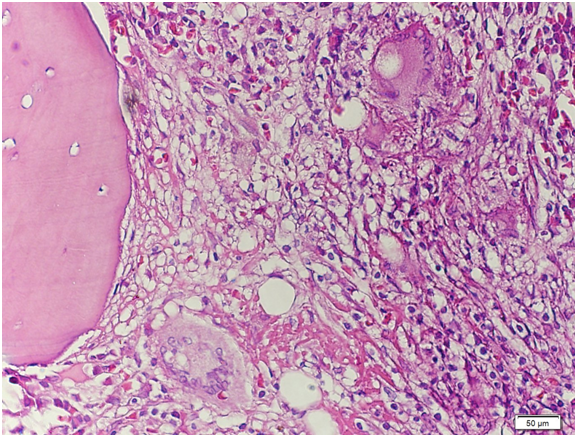
Figure 14 High‒power view. There is significant granuloma formation with Langhans type giant cell (arrows) focally scattered in the marrow matrix. The residual marrow shows erythrogranulopoiesis with maturation. Megakaryocytes are adequate.
A bone marrow aspirate and core biopsy was done in our patient primarily to rule out a hematologic disorder to explain the hematologic signs and symptoms of the patient. Several researches showed the utility of bone marrow aspirate and a blood culture to diagnose disseminated tuberculosis. Some investigators have used PCR to detect mycobacterial DNA in bone marrow smear and it is reported to be useful.15 Bone marrow aspiration however is an invasive procedure, and several studies show that blood culture compares to bone marrow biopsies in its yield to diagnosis disseminated tuberculosis. In the study by Crump & Reller,23 mycobacterial blood culture appeared to be as sensitive as bone marrow culture in diagnosing disseminated tuberculosis (sensitivity, 58% vs. 54%). Mycobacterial blood culture can play an increasing role in the diagnosis of disseminated tuberculosis when localized disease is not found. Comparing bone marrow biopsies with blood cultures in the recovery of mycobacteria in the diagnosis of disseminated tuberculosis, Pacios et al.24 noted that blood culture represent a more sensitive and less invasive alternative to bone marrow cultures, but provided no advantage for the diagnosis of disseminated mycobacterial infection caused by Mycobacterium tuberculosis.24 To diagnose disseminated tuberculosis, a search for sites of localized disease should be undertaken, and samples from these sites should be obtained. Examination of biopsy specimens from sites of localized disease, especially lymph nodes, has a high diagnostic yield, including bone marrow biopsies and blood culture. Combined mycobacterial culture and histopathologic examination of biopsy from bone marrow were more sensitive and faster than mycobacterial blood culture in diagnosing disseminated tuberculosis.
We have presented a case of disseminated tuberculosis with the unusual presentation of hematologic derangements with onset of medications for tuberculosis. It is important to differentiate adverse reactions due to the medications from a primary hematologic disorder. Non‒improvement of symptoms upon cessation of medications and a bone marrow biopsy is useful in differentiating between the two. Bone marrow biopsies are also useful in diagnosing disseminated tuberculosis especially when diagnosis is difficult. Blood culture may also be an alternative, as it is also less invasive. As disseminated tuberculosis may present with several life‒threatening hematologic disorders, prompt diagnosis is crucial.
None.
None.

©2016 Madamba. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.