eISSN: 2377-4304


Research Article Volume 4 Issue 6
Department of Obstetrics and Gynecology, Ain Shams University Maternity Hospital, Egypt
Correspondence: Mohammed El-Sokkary, Assistant professor of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Abbasyia, Cairo, Egypt
Received: July 08, 2016 | Published: July 21, 2016
Citation: El-Sokkary M, Shafik A, Nader S. The Role of Endometrial Volume in the Prediction of Endometrial Hyperplasia. Obstet Gynecol Int J. 2016;4(6):228-231. DOI: 10.15406/ogij.2016.04.00136
Objective: To assess the accuracy of 3D Trans vaginal sonar (TVS) in the diagnosis of endometrial hyperplasia in women with premenopausal uterine bleeding.
Methods: Seventy-five women with premenopausal bleeding were recruited from outpatient gynecology clinic in Ain Shams University Maternity Hospital. The study was conducted from May (2014) till January (2015). The endometrial volume obtained by 3D TVS and results of the histopathological examination of the endometrial tissue were evaluated to assess the cut off value of endometrial volume for diagnosis of endometrial hyperplasia and carcinoma.
Results: In the current study, the histopathological results showed 15 cases with atrophic endometrium (20%), 7 cases with disordered proliferative endometrium (9.33%), 7 cases with hyper plastic polyp (9.33%), 14 cases with endometrial hyperplasia without atypia (18.67%), 3 cases with complex endometrial hyperplasia without atypia (4%), 3 cases with hyperplasia with atypia (4%) and 26 cases with adenocarcinoma (34.67%). The median endometrial volume of benign endometrium was 9.6 with interquartile ratio (7.1-12.5) while the median endometrial volume of endometrial hyperplasia was 11.4 with interquartile ratio (7.4-14.7) and the median endometrial volume of endometrial carcinoma was 17.26 with interquartile ratio (14.9-24.1). The difference was highly statistically significant using Kruskal-Wallis test.
Conclusion: Endometrial hyperplasia is the commonest observed endometrial abnormality in premenopausal patients with abnormal uterine bleeding. Even though histopathological examination of the endometrium is the gold standard for diagnosis or exclusion of endometrial pathology, 3D TVS is reasonably accurate, helpful & non-invasive tool for assessing the endometrium.
Keywords:Endometrial volume, Endometrial hyperplasia
Premenopause is a transitional period 3-5 years prior to menopause that is usually characterized by a change in the normal menstrual cycle. The cycles may be longer or shorter, and the flow may vary from light to heavy. As ovarian function is declining, ovulation may not occur. The unopposed estrogen without progesterone will cause the uterine lining to thicken.1 This thickening will cause endometrial hyperplasia & carcinoma, polyps & fibroids may also cause changes in bleeding pattern.2 Endometrial sampling is the gold standard for diagnosing abnormalities in the endometrial tissues with sensitivity ranging from 85-95%.3 There is a growing trend to use noninvasive procedures such as TVS, to measure the endometrial thickness, diagnose dysfunctional uterine bleeding, adenomyosis, endometrial polyps & leiomyomas.4 Another important ability of 3D TVS is volume calculation using the Virtual Organ Computer-aided AnaLysis (VOCAL) even in irregularly shaped structures. This method has been demonstrated to be more accurate than 2D-volume estimation.5 The differentiation between benign endometrial pathology, endometrial hyperplasia and carcinomas was not possible due to overlap in the endometrial thickness measurements. When 3D volume measurements were performed, the overlap was much smaller which significantly improved the diagnosis of cancer.6 The purpose of this study was to assess the accuracy of endometrial volume estimation by 3D TVS in diagnosis of endometrial hyperplasia in women with premenopausal abnormal bleeding.
This study was conducted in a single tertiary care academic center (Ain Shams University Maternity Hospital). from May 2014 till January (2015), 75 women who sustained premenopausal bleeding were enrolled in this study.
All patients were subjected to the following
Ultrasound instrumentation
With an empty bladder, the patient was examined at the Special Care Center of The Fetus by the same sonographer in the lithotomy position using Voluson Pro 720 transducer with frequency range 5-8 MHZ. Sonar is done to study uterine size, shape and exclusion of any uterine or ovarian pathology. The 3D image was obtained by switching on the 3D volume mode and defining the region of interest by a movable sector on the screen. This sector has the shape of a truncated cone which can be manipulated to ensure that the whole of the endometrial cavity was included in the volume sampling while the patient remain still and the probe is held stationary. Volume sampling lasted about 4 seconds, during that time the conventional 2D plane was rotated through 180ᵒ with the rotation axis oriented exactly along the longitudinal axis of the vaginal probe. The data was stored digitally on the internal disc drive for subsequent analysis after the ultrasound probe is removed. For the purpose of volume calculation, 3D data was retrieved and presented in multi-planer display mode which simultaneously displays 3 perpendicular planes on the screen. The actual volume was calculated by the built-in computer program using VOCAL. This is a rotational method based on rotation in given steps (6, 9, 15, 30) on a given orthogonal plane (A, B OR C). The endometrial volume was measured in plane A by delineating the endometrial margin at the endometrial-myometrial interface from the fundus to the internal cervical Is in a number of parallel slices which are 1-2 mm apart.
Endometrial curettage
Endometrial curettage was performed using a sharp-ended curette under general anesthesia by the same surgeon. The first sample was taken from the end cervical canal before cervical dilatation, then cervical dilatation up to 7-8 Hegar. A sharp curette is introduced and curettage starting first with the fundus then posterior wall then anterior wall then right then left lateral walls. The sample was placed in Formalin 10% and sent for histopathological examination for the nature of the endometrial pathology which was done in Early Cancer Detection Unit at ASUMH.
Blind comparison
Sonographer who undergoes 3D TVS was unaware of the result of the histopathological examination of the endometrial tissue. And the pathologist who undergoes the histopathological examination was not aware of the result of 3D TVS.
Ethical consideration
Institutional review board (IRB) approval: The protocol was discussed by the ethical scientific committee for approving the study and informed consent was obtained before participation.
Consent procedure
The Investigator made certain that an appropriate informed consent process was in place to ensure that potential research subjects, or their authorized representatives, were fully informed about the nature and objectives of the clinical study, the potential risks and benefits of study participation, and their rights as research subjects. The Investigator obtained the written, signed informed consent of each subject, or the subject’s authorized representative, prior to performing any study-specific procedures on the subject. The Investigator retained the original signed informed consent form.
Subject confidentiality
All laboratory specimens, evaluation forms, reports, video recordings, and other records that leave the site would not include unique personal data to maintain subject confidentiality.
Sample size calculation
The required sample size has been estimated using the Power Analysis and Sample Size software version 08.0.9 (PASS; NCSS; LLC; Kaysville, Utah). The test used for calculation is the two sided z-test and type 1 error has been set at a two sided value of 0.05 (confidence level, 95%).
The purpose of this study was to assess the accuracy of 3D Trans-vaginal Ultrasound in the diagnosis of endometrial hyperplasia by endometrial volume measurement in women with premenopausal bleeding. The current study was conducted in Ain Shams University Maternity Hospital on women recruited from the outpatient gynecological clinic from May (2014) till December (2014). 75 premenopausal women presented by abnormal uterine bleeding were included in the study. Women with benign endometrial pathology were 22 (29.33%), which included atrophic and disordered proliferative endometrium. Women with endometrial hyperplasia were 27 (36%) which included hyper plastic polyp, Simple Endometrial hyperplasia without atypia and others with atypia and complex endometrial hyperplasia while endometrial carcinoma was found in 26 (34.67%) patients (Table 1 & Figure 1).
|
Histo pathological result |
Number (%) |
|
Atrophic Endometrium |
15 (20) |
|
Disordered Proliferative Endometrium |
7 (9.33) |
|
Hyperplastic Polyp |
7 (9.33) |
|
Simple Endometrial Hyperplasia without Atypia |
14 (18.67) |
|
Complex Endometrial Hyperplasia |
3 (4) |
|
Simple Endometrial Hyperplasia with Atypia |
3 (4) |
|
Adenocarcinoma |
26 (34.67) |
Table 1 The distribution of the history pathological results in women under study
There was a statistically significant difference between benign endometrium compared to endometrial hyperplasia and to endometrial carcinoma as regard age [(49.6±2.6, 51.7±2.8 and 51.7±2.7) respectively] and parity [4 (3 – 5), 3 (2 – 4) and 2 (1 – 3) respectively] using Independent sample t-test but there was no significant difference among the groups as regards weight and occurrence of previous abortions. Also, there was a highly statistically significant difference between patients with benign endometrium, endometrial hyperplasia and endometrial carcinoma, as regarding endometrial volume [9.6 (7.1-12.5), 11.4 (7.4-14.7) and 17.2 (14.9-24.1) respectively], using Kruskal-Wallis test (Table 2 & Figure 2).
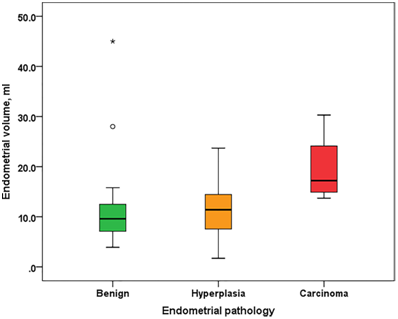
Figure 2 Box plot showing endometrial volume in patients with benign endometrial pathology, endometrial hyperplasia, or endometrial carcinoma. Box represents the range between the 1st and 3rd quartiles (interquartile range). Horizontal line inside the box represents the median. Whiskers represent minimum and maximum values excluding outliers (rounded markers) and extreme observations (asterisks).
|
Benign endometrial pathology (n=22) |
Endometrial hyperplasia (n=27) |
Endometrial carcinoma (n=26) |
p-value |
|
|
Age, yr |
49.6±2.6 |
51.7±2.8† |
51.7±2.7† |
0.014 |
|
BMI kg/m2 Mean±SD |
33.4±6.1 |
35.4±5.0 |
35.1±5.3 |
0.373 |
|
Parity |
4 (3 – 5) |
3 (2 – 4) |
2 (1 – 3)‡ |
0.026 |
|
Previous abortions |
0 (0 – 1) |
0 (0 – 1) |
1 (0 – 1) |
0.562 |
Table 2 Characteristics of patients with benign endometrial pathology, endometrial hyperplasia, or endometrial carcinoma
It was shown by the results of receiver-operating characteristic (ROC) curve analysis for classification of patients into those with benign endometrial pathology and those with endometrial hyperplasia or carcinoma using endometrial volume that endometrial volume had a good predictive value as evidenced by an area under the ROC curve (AUC) of 0.708 (95% CI, 0.572 to 0.843). The best cut-off value was an endometrial volume >13.2 ml (Youden index J, 0.497). This had a sensitivity of 67.9% (95% CI, 53.7% - 80.1%), a specificity of 81.8 (95% CI, 59.7% - 94.8%), a positive predictive value (PPV) of 90.0% (95% CI, 76.3% - 97.2%), and a negative predictive value (NPV) of 51.4% (95% CI, 34.0% - 68.6%) (Table 3).
|
Index |
Estimate (95% CI ( |
|
Area under the ROC curve (AUC ( |
0.708 (0.572 to 0.843) |
|
P-value (AUC0=0.5) |
0.003 |
|
Youden Index J |
0.497 |
|
Best cut-off value, ml |
>13.2 |
|
Sensitivity % |
67.9 (53.7 - 80.1) |
|
Specificity % |
81.8 (59.7 - 94.8) |
|
Positive predictive value (PPV %) |
90.0 (76.3 - 97.2) |
|
Negative predictive value (NPV %) |
51.4 (34.0 - 68.6) |
Table 3 shows the results of receiver-operating characteristic (ROC) curve analysis for classification of patients into those with benign endometrial pathology and those with endometrial hyperplasia or carcinoma using endometrial volume
Also, the results of receiver-operating characteristic (ROC) curve analysis for classification of patients into those with benign endometrial pathology or hyperplasia and those with endometrial carcinoma using endometrial volume had shown that endometrial volume had a very good predictive value as evidenced by an area under the ROC curve (AUC) of 0.861 (95% CI, 0.777 to 0.945). The best cut-off value was an endometrial volume >13.5 ml (Youden index J, 0.755). This had a sensitivity of 100% (95% CI, 86.8% - 100.0%), a specificity of 75.5% (95% CI, 61.1% - 86.7%), a positive predictive value (PPV) of 68.4% (95% CI, 51.3% - 82.5%), and a negative predictive value (NPV) of 100% (95% CI, 90.5% - 100.0%) (Table 4).
|
Index |
Estimate (95% CI ( |
|
Area under the ROC curve (AUC ( |
0.861 (0.777 to 0.945) |
|
P-value (AUC0=0.5) |
<0.0001 |
|
Youden Index J |
0.755 |
|
Best cut-off value, ml |
>13.5 |
|
Sensitivity % |
100 (86.8 - 100.0) |
|
Specificity % |
75.5 (61.1 - 86.7) |
|
Positive predictive value (PPV %) |
68.4 (51.3 - 82.5) |
|
Negative predictive value (NPV %) |
100 (90.5 - 100.0) |
Table 4 Receiver-operating characteristic (ROC) curve analysis for classification of patients into those with benign endometrial pathology and those with endometrial hyperplasia or carcinoma using endometrial volume at a cut off value of 13.2 ml
Heavy menstrual bleeding is a major public health problem.7 Menstrual disorders interfere significantly with the quality of life in otherwise healthy women. Whenever bleeding occurs, judgment is needed to determine whether investigation is required to rule out benign and malignant causes.8 Abnormal uterine bleeding is probably the most common symptom in gynecologic practice. Up to 33% of women referred to the gynecological outpatient clinics have abnormal uterine bleeding and this proportion rises to 69% in the premenopausal group.9 Traditionally, dilatation and curettage used to be the main line of investigation for abnormal uterine bleeding but it is not accurate for diagnosing focal intrauterine lesions which are small or located in areas difficult to curette.10 Also, in studies comprising both pen and postmenopausal women with abnormal uterine bleeding, 43-66% of eases of hyperplasia were missed by D&C.11
Trans vaginal 2D ultra sonography has been used extensively in cases of abnormal uterine bleeding to evaluate uterine pathology to exclude myomata, polyps and focal lesions and to cheek the adnexa.12 3D ultrasound offers new viewing window by allowing for arbitrary plane evaluation through a volume data set acquired from the pelvis12. In addition, by 3D ultrasound, more precise anatomical sections for exploring the endometrial cavity; the relations of myomata and their possible encroachment on the cavity, the diagnosis of endometrial polyps and the measurement of endometrial volume rather than thickness in cases of abnormal uterine bleeding are feasible.13 The incidence of endometrial cancer has increased in the past decade although the incidence and mortality rates of other types of cancer have stabilized or even decreased.14
In the current study there was a statistically significant difference as regards the mean age between women with benign endometrial pathology compared to that of either endometrial hyperplasia or carcinoma are (49.6±2.6, 51.7±2.8 and 51.7±2.7 respectively). In disagreement with our results, the study done by Clark et al.15 and Ahmad et al.9 who found no statistically significant difference between the study groups.
In this study the histopathological results shows 15 women with atrophic endometrium (20%), 7 women with disordered proliferative endometrium (9.33%), 7 women with hyper plastic polyp (9.33%), 14 women with endometrial hyperplasia without atypia (18.67), 3 women with complex endometrial hyperplasia (4%), 3 women with endometrial hyperplasia with atypia (4%), 26 women with adenocarcinoma (34.67%). This is in contrast to another study which showed that normal endometrium was found in 9 cases (18%), myomas in 16 cases (32%), endometrial polyps in 6 cases (12%), endometrial hyperplasia in If cases (22%) and endometrial carcinoma in 2 cases (4%).10 The prevalence of endometrial carcinoma in the present study was 34.67%. This is similar to that reported in previous studies.16,17 It is author`s opinion that the incidence of endometrial carcinoma in Egypt is more than others and this is according to many factors as obesity which has high incidence & low compliance for treatment & seeking medical advice late after the disease is late in stage but this is still under study by the authors.
As regards endometrial volume, the difference between patients with benign endometrium, compared to endometrial hyperplasia and endometrial carcinoma was highly statistically significant, the medians (IQR) were [9.6 (7.1-12.5), 11.4 (7.4-14.7) and 17.2 (14.9-24.1) respectively, using Kruskal-Wallis test. Also, the difference between patients with benign endometrial pathology and endometrial hyperplasia compared to endometrial carcinoma was statistically significant. In another study,18 the endometrial volume in hyperplasia had the mean value of 7.82 ± 7.60 cc and was significantly higher than the volume ha patients with polyps (mean 2.63 ± 2.12 cc). This was not found in the present study. In another study19 was done on premenopausal patients, the endometrial volume was 6.87± 6.3 cc in the normal group and 13.79 ± 13.2 cc in the pathologic group. Endometrial volume was 18.1 cc in patients with endometrial cancer and 11.2 cc in patients with hyperplasia; both were significantly higher than in the normal. In the study by Stachowicz et al.,20 the mean endometrial volume in women with endometrial cancer was 19.9 ± 7.5 cc. The mean volumes measured in women with endometrial hyperplasia and normal endometrium, were 12.2 ±7.9 cc and 7.4 ±4.8 cc, respectively.
In the current study (ROC) curve analysis for classification of patients into those with benign endometrial pathology and those with endometrial hyperplasia or carcinoma using endometrial volume Endometrial volume had a good predictive value as evidenced by an area under the ROC curve (AUC) of 0.708 (95% CI, 0.572 to 0.843). The best cut-off value was an endometrial volume >13.2 ml (Youden index J, 0.497). This had a sensitivity of 67.9% (95% CI, 53.7% - 80.1%), a specificity of 81.8 (95% CI, 59.7% - 94.8%), a positive predictive value (PPV) of 90.0% (95% CT, 76.3% - 97.2%), and a negative predictive value (NPV) of 5l.4% (95% CT, 34.0% - 68.6%).
While (ROC) curve analysis for classification of patients into those with benign endometrial pathology or hyperplasia and those with endometrial carcinoma using endometrial volume; Endometrial volume had a very good predictive value as evidenced by an area under the ROC curve (AUC) of 0.861 (95% CT, 0.777 to 0.945). The best cut-off value was an endometrial volume >13.5 ml (Youden index J, 0.75 5). This had a sensitivity of 100% (95% CT, 86.8% - 100.0%), a specificity of 75.5% (95% CI, 61.1% - 86.7%), a positive predictive value (PPV) of 68.4% (95% CT, 5 1.3% - 82.5%), and a negative predictive value (NPV) of 100% (95% CT, 90.5% - 100.0%).
Ebrashy et al.21 examined 65 cases by both TVS 2D and 3D ultrasound and the results are by 2D ultrasound: 13 cases are normal, 7 cases showing endometrial polyps, 29 cases having myomas either single or multiple from which 8 had sub mucus myomas, 12 cases had thickened endometrium while by 3D ultrasound: 9 cases are normal. In the study carried out by Pyrai et al.10 on 50 patients with abnormal uterine bleeding, by TVS it detected 13 myomas (26%), 4 polyps (8%), 3 adenomyosis (6%), 10 hyperplasia (20%), 2 endometrial carcinoma (4%), 2 atrophic endometrium (4%).10
While in another study which dealt with similar parameters to detect the value of sonography in relation to hysteroscopy in diagnosing intracavitary lesions showed sensitivity for 2D U/S of 74% for myomas and 39% for polyps while sensitivity of hysteroscopy is 100% for myomas and 99% for polyps.22 Also Dery et al.23 revealed the sensitivity of hysteroscopy for endometrial polyps is 94%, specificity 92% and sensitivity for sub mucus myomas 87% and specificity 95% with overall sensitivity for assessment of the uterine cavity 96% and specificity 90%.23 Bonnamy et al.24 showed sensitivity and specificity of 2D U/S in diagnosis of fibroids is 65% and 94% respectively while that of hysteroscopy is 88% and 94% respectively.24
A prospective study carried on 56 patients by showed sensitivity, specificity, positive predictive value, negative predictive value of 3D U/S in diagnosis of fibroids is 84.8%, 79%, 82.4%, and 82% respectively. TVS has a high sensitivity for detecting myomas in a uterus <10 weeks size. The use of high frequency probes improves the sensitivity for diagnosing small myomas although their precise location with respect to the uterine cavity often remains uncertain. Hysteroscopy is accurate but invasive in evaluating uterine mypmata25
In another study evaluated the accuracy of endometrial volume measurement in the diagnosis of endometrial carcinoma and endometrial hyperplasia in 89 women with premenopausal bleeding. All were scheduled for hysteroscopy, dilatation and curettage, endometrial sampling or hysterectomy and the ultrasound was performed within 24 h before the procedure. Endometrial volume was measured and compared between the groups of women with different histopathology. 17.9% of patients had an endometrial polyp, 12.5% had hyperplasia and 7.6% had endometrial carcinoma. The mean endometrial volume was 6.87 cc, 5.43 and 15.5 cc respectively (p <0.001). Concluding that endometrial volume is a good diagnostic tools a predicting endometrial carcinoma and hyperplasia in women with premenopausal bleeding.19
Another study was done in 2007 by Mansour and co-workers26 to assess endometrial volume as a predictor of endometrial malignancy in women with postmenopausal bleeding. Endometrial volume was measured by VOCAL in a group of women with postmenopausal bleeding. Another group of women without postmenopausal bleeding was used for control. 50% of cases in the study group had benign disease, 35% had atypia and 15% had cancer. Whereas endometrial thickness was 9.61+1-5.12 mm (range, 5-20 mm) and endometrial volume was 3+1-1.1 mL (range, 1.8-5.4 mL) in women with atypia or cancer, they were 4.87+7-3.43 mm (range, 2-8 mm) and 1.52±0.82 (range, 0.6-2.2 mL), respectively, in women with benign disease. In the control group, endometrial volume was 1.15+/-0.14 mL (range 0.6- 1.3 mL). Volume was more sensitive than thickness for predicting malignancy, and a cutoff value of 1.35 mL was found to provide the best sensitivity.26
Conclusion of this study stated that endometrial hyperplasia is the commonest observed endometrial abnormality in premenopausal patients with abnormal uterine bleeding. Even though histopathological examination of the endometrium is the gold standard for diagnosis or exclusion of endometrial pathology, 3D ultrasound is a reasonably accurate, helpful and non-invasive tool for assessing the endometrium. An endometrial volume of 13.5 mL or greater may predict malignancy in women with premenopausal bleeding.
None.
None.

©2016 El-Sokkary, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.