eISSN: 2377-4304


Case Report Volume 4 Issue 3
1Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery (MUAPOS), University General Hospital of Castellon, Spain
2Department of Medicine, University Jaume I, Spain
Correspondence: Herraiz Roda Jose Luis, University General Hospital of Castellon, Av Benicasim s/n, 12004 Castelln, Spain
Received: October 23, 2015 | Published: February 24, 2016
Citation: Luis HRJ, Llueca AA, Piquer SD, et al. Ovarian neuroendocrine carcinoma-case report. Obstet Gynecol Int J. 2016;4(3):80-82. DOI: 10.15406/ogij.2016.04.00106
This paper refers to a case of high‒grade ovarian tumor with neuroendocrine differentiation in University General Hospital of Castellon. The gynaecologic literature refers to this tumor an incidence less than 0.1% of all ovarian tumors, and represents nearly 3% of all neuroendocrine tumors. We present the case of a 70‒year‒old woman who presented an ovarian neuroendocrine carcinoma with associated carcinomatosis.
Keywords: Neuroendocrine tumor, Ovarian tumor, Carcinoid syndrome, Citoreduction surgery
Ovarian carcinoid tumors are rare neoplasms that may be primary or metastatic. The gynaecologic literature refers to this tumor an incidence less than 0.1% of all ovarian tumors,1 and represents nearly 3% of all neuroendocrine tumors.2 Primary ovarian carcinoid tumors are usually unilateral, localized to the ovary, and composed of gastrointestinal or respiratory epithelium, they often arise within a cystic teratoma or dermoid tumor. 57% of primary ovarian carcinoids coexisted with cystic teratomas/dermoid tumors.3
Ovarian neuroendocrine carcinoma can be small cell carcinoma (SCNEC) of hypercalcemic or pulmonary type or non small cell carcinoma (NSCNEC). Both of them are rare and aggressive tumours and can be admixed with other surface epithelial and germ cell ovarian neoplasm. NSCNEC is presented in postmenopausal women and symptoms are related with a pelvic or abdominal mass: pain, ascites, vaginal bleeding and abdominal bloating.4 The overall prognosis is poor, they are currently presented at advanced stage.
In a review of medical histories of oncological patients taken control in the gynecology service of the University General Hospital of Castellon during the last 5 years, we only have found this clinical case of neuroendocrine ovarian carcinoma. Owing to the paucity of cases in the literature and to its low incidence, we have decided to publish it.
A 70‒year‒old woman was referred by internal medicine, in April 2013, because her physical examination revealed ascites and a mass in the lower abdomen. She had a personal history of cerebrovascular disease and atrial fibrillation. Complementary tests were done to filiate and stage the lesion. Transvaginal ultrasound showed an atrophic utero and enlarged ovaries with low resistance flow and much free fluid within the abdomen. Magnetic resonance imaging showed bilateral carcinoma in the ovaries that together presented dimensions of 11.5x6x7.5 cm (transverse, craniocaudal and anteroposterior), many free fluids and a possible metastatic diaphragmatic nodule. CT scan informed about bilateral pleural effusion and prevascular mediastinal lymph nodes (Figure 1).
In the diagnostic laparoscopy we extracted 6 liters of ascitic fluid. The ovaries were enlarged (malignant aspect) and the pelvis was frozen, there were multiple peritoneal implants in pelvis and diaphragm: biopsies were taken. The peritoneal carcinomatosis index (PCI) was 20.
The histopathology showed ovarian and peritoneal invasion by high‒grade carcinoma with neuroendocrine differentiation. Pathology examination showed a tumour composed of two different components, the larger of them was characterized by a dense population of medium ovoid and fusiform cells, arranged in solid nest and trabeculae with central necrosis (Figure 2). The tumours cells have scant cytoplasm and hyperchomatic nucleus with “salt and pepper” chromatin. The smaller component consisted of papillary and glandular epithelial structures, with severe atypia and frequent Psammoma bodies (Figure 3). These features were characteristic of non small cell neuroendocrine carcinoma (NSCNEC) plus High‒Grade serous carcinoma.
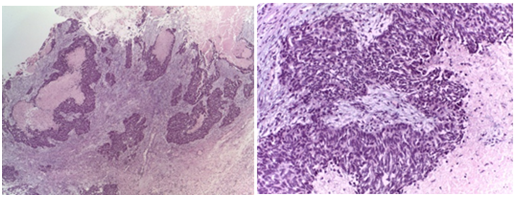
Figure 2 Low power view of neuroendocrine carcinoma showing solid nest and trabeculae with necrosis.
Immunohistochemical stains showed neuroendocrine markers (CD56, synaptophysina and neuronal specific enolase) and keratin 7 in the first component and Wilm´s tumor‒1 (WT‒1) (Figure 4), keratin 7 and Pan‒keratin in the serous carcinoma component. Both of them were negative for estrogen receptor (ER), progesterone receptor (PR) and thyroid transcription factor (TTF‒1).
In May 2014, the committee of gynecological tumors decided to send the patient to the medical oncology service for neoadjuvant therapy with carbetopoxid given in three cycles. CA 125 and NSE serum level reduced, pleural effusion disappeared, and ascites decreased. There were no significant changes in ovaries size.
Five months from the first diagnosis and after bibliographical reviews and several meetings with the tumors committee, we decided to do citorreduction surgery. The peritoneal carcinomatosis index (PCI) was 20 in the laparotomy and we achieved a citorreduction coefficient of 0 (CC0) after surgery. During the post‒surgery period the dehiscence of ileocolic and colorectal anastomosis complicated the clinical course with ileostomy. The patient evolved satisfactorily.
Adjuvant chemotherapy was carried out with cisplatin and etoposide. In June 2015 PET/CT scan revealed suprarenal, liver, axillar and spleen nodules. The patient was given second‒line chemotherapy.
Neuroendocrine tumors come from APUD system cells (amine precursor uptake and decarboxylation). Most of them are sporadic and they present neither a cause nor a few concrete risk factors well known. Nevertheless, some cases can appear in hereditary syndromes. They are characterized by germ line mutations usually inherited as an autosomal dominant disease such as Multiple Endocrine Neoplasias (MENs).
These tumors are typically classified as germ cell tumors of the ovary and can be divided into four categories:
i. Insular,
ii. Trabecular,
iii. Mucinous, and
iv. Mixed
Primary carcinoid tumors typically behave in a benign fashion. Most ovarian carcinoids contain the insular pattern, are unilateral and early stage. Strumal carcinoid has only recently been recognized as a distinct entity as thyroid tissue with carcinoid tumor. It is important to establish that these are not metastatic carcinoids, the insular, followed by the trabecular are the most common subtypes that metastasize to the ovary.5‒8 The majority of women with primary ovarian carcinoid tumors are found incidentally in a surgery or ultrasound imaging. In our patient the tumor has been found in an advanced phase in a context of increased abdominal girth. Rarely, they may also present a carcinoid syndrome with abdominal pain, constipation, hirsutism, erythema, tachycardia and sweating.9 There are symptoms caused by vasoactive substances.
The constipation and hirsutism are thought to be due to the release of Peptide YY (PYY) by the tumors. PYY is a gastrointestinal peptide that is present mainly in the endocrine cells of the distal intestine and inhibits motility.9
In Davis & Talerman publications.10,11 they compare the incidence of the carcinoid syndrome depending on its origin and coincide that the symptoms are more frequent if the tumor is in the ovary because its drainage goes directly to the vena cava without having hepatic step that would inactivate big part of vasoactive substances.3 Surgery appears to be the treatment of choice in early stages.9 In our case, first we started with neoadjuvant treatment due to the high‒grade of carcinomatosis that the patient presented in diagnostic laparoscopy. The tumors committee considered surgery as the only treatment option due to low response to chemotherapy. Davis10 recounts survival of 100 % to 5 years, in the patients with illness confined in the ovary, being the salpingo‒oophorectomy the surgery best choice.9 Nowadays our patient has a survival rate of 27 months despite of having an advanced stage.
In advanced stages a combination of radiation therapy.12 and chemotherapy may be recommended. However, only a few cases have been published to date.1
None.
None.

©2016 Luis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.