eISSN: 2377-4304


Research Article Volume 2 Issue 2
Fernandes Figueira National Institute of Women, Children and Adolescents Health, Brazil
Correspondence: Valeria Seidl, Estrada dos Bandeirantes 28.600 casa 23. Rio de Janeiro-RJ, Brazil, Tel (21) 99415-8694
Received: December 17, 2014 | Published: April 13, 2015
Citation: Moreira MEL, Filho FMP, Moura Sa CA, et al. Risk factors related to severe outcome in RhD hemolytic disease of the fetus and newborn. Obstet Gynecol Int J. 2015;2(2):65-69. DOI: 10.15406/ogij.2015.02.00033
Objective: To determine the major risk factors related to exchange transfusion in pregnancies afflicted with RhD hemolytic disease of the fetus and newborn (HDFN).
Methods: A cohort study of 124 infants born with this disease between April 2006 and June 2009 at the Fernandes Figueira Institute. Data on maternal history, prenatal care, delivery and neonatal parameters were subjected to univariate analysis to determine their relationship to severe disease outcome, represented by the need for exchange transfusion. Significant variables were subjected to multivariate and multi level analysis.
Results: Diverse simulations were performed in the model to choose the most representative ones. As final result, intrauterine transfusion (RR: 2,54 [1,64-4,00]), jaundice (RR: 2,77 [1,76-4,39]), peak serum bilirubin levels during hospitalization (RR: 1,16 [1,11-1,22]), were the variables found to establish an independent and statistically significant relationship with severe outcome.
Conclusion: Recognition of risk factors for exchange transfusion in pregnancies afflicted with RhD HDFN is possible and necessary regarding newborn care following delivery.
Keywords: Rh alloimmunization, Risk factors, Hemolytic disease of the fetus and newborn, Exchange transfusion
HDFN, hemolytic disease of the fetus and newborn; DAT, direct antiglobulin test; RR, relative risk; IVIg, intravenous immunoglobulin.
Hemolytic disease of the fetus and newborn (HDFN) is a condition in which specific maternal antibodies that cross the placenta reduce the lifespan of fetal red blood cells, causing fetal anemia. Prevalence of RhD disease in the United States was 6.8/1,000 births.1 In Brazil, official data are scarce because of the characteristics of the Hospital Information System of the Brazilian National Unified Health System, which led to underreporting.2 Data calculated from the frequency of blood groups and from ABO and Rh(D) incompatibilities suggest that 10% of all pregnant women are Rh(D)-negative, and 70% of these women conceive Rh(D)-positive children.3
Since the implementation of protocols that advocate the use of antenatal prophylaxis with anti-D immunoglobulin around the 28th week of pregnancy and up to 72 h postpartum, the disease outcome has undergone great changes, as shown by the swift and significant decrease in cases of maternal alloimmunization.4 Currently, the incidence rates have been constant because of other factors, such as failures in immunoprophylaxis administration (absent, insufficient or untimely), the failure to recognize clinical episodes of maternal-fetal hemorrhage, incompatible blood transfusion and spontaneous sensitization.5
In developing countries with faulty maternal immunization programs, stillbirth occurs in 14% of the afflicted pregnancies, while 50% of the fetuses who are alive at birth do not survive the neonatal period or develop brain damage.6
Adequate care requires the screening of Rh(D)-negative pregnant women, early recognition of those at risk of developing HDFN and the use of adequate prophylaxis for nonsensitized pregnant women.7 Early referral of sensitized pregnant women to receive adequate prenatal care allows appropriate control of fetal anemia development enabling intrauterine transfusion therapy when necessary. The treatment of infants with HDFN represents a complex process carried out with pediatric assistance; requiring, admission to a neonatal intensive care unit, need for phototherapy, availability of Rh-D negative screened blood type, and technical expertise for exchange transfusion.8,9 These health care procedures are needed in order to avoid severe child anemia and jaundice, leading to a better neonatal outcome.
We therefore consider that identification of risk factors during prenatal and early neonatal care would be useful in the clinical management of the main complications related to HDFN. The present work aimed to assess which characteristics of maternal history, prenatal care, delivery and newborn care are most strongly related to severe neonatal outcomes, defined here as the need for exchange transfusion after birth.
A cohort study was performed to assess the babies born from April 2006 to June 2009 that involved those newborn infants with hemolytic disease caused by Rh(D) incompatibility. For this purpose, we monitored the pregnancy and delivery of women diagnosed with Rh alloimmunization at the FernandesFigueira National Institute. Access to prenatal care can occur at any gestational age, and the births take place at the institute itself, where the infants are followed at an outpatient clinic that specializes in the first year of life. Patients with fetal malformation and hydrops resulting from causes other than anti-D alloimmunization were excluded from the study. Data regarding the selected women’s variables of interest and data on the delivery and the newborn infant were obtained from medical records. This study was approved by the Human Been Research Ethics Committee of the FernandesFigueira Institute/FIOCRUZ and complies with the current guidelines for research on human beings established in resolution No. 196/96 of the Brazilian Ministry of Health.
In order to assess whether an effect was direct or mediated through other factors, variables included in this study were divided into three groups, according to the association level with the outcome (distal, intermediate and proximal) (Table 1).
Maternal history and Prenatal care |
Delivery |
Newborn |
|---|---|---|
1 HDFN history |
11 Vaginal delivery |
16 Anemia |
2 Knew Rh- |
12 Suffering |
17 Jaundice |
3 Had RhoGAM |
13 Oxygen use |
18 Complications |
4 Type A |
14 IMV |
19 Preterm |
5 Type AB |
15 5-minute Apgar |
20 Birth weight |
6 Type B |
|
21 Peak BL |
7 Hydrops |
|
22 Hematocrit |
8 Had MCA |
|
23 Direct Coombs |
9 Had IUT |
|
|
10 PN IFF |
|
|
Table 1 Risk factors variables divided into three groups, according to the association level with the outcome in a multivariate model analysis
The first group referred to maternal history and prenatal care (distal level)
Maternal age, parity, blood type (ABO, Rh factor, phenotype), obstetric history related to RhD disease (stillbirth or neonatal death, hydrops, intrauterine transfusion therapy, exchange transfusion), maternal transfusion, use of drugs, whether the patient underwent immunoprophylaxis in a previous pregnancy, whether she knew that she was Rh(D)-negative and whether she knew the condition could harm her child, when prenatal care began (months), the gestational age at which prenatal care was initiated at the InstitutoFernandesFigueira (weeks), paternal blood type (ABO, Rh factor, phenotype), whether the father was the same as for previous pregnancies, indirect antiglobulin test of the mother, type of immunoglobulin present in the maternal serum at birth (IgG levels and subclasses 1 and 3, determined via direct antiglobulin test (DAT) IgG-Dilution and DAT IgG1/IgG3 DiaMed® cards), ultrasound diagnosis of hydropsfetalis (ascites, regardless of association with serous effusions and subcutaneous edema), whether fetal anemia was monitored via Doppler velocimetry of middle cerebral artery peak systolic velocity measurements establishing gestational age and the values of the first and last fetal evaluation, the need for intrauterine transfusion therapy (how many), gestational age and hematocrit and hemoglobin levels at the first and last transfusion, transfused volume, the occurrence of complications and the puncture site.
The second group of variables (intermediate level) referred to delivery
Type of delivery (vaginal or cesarean section); complications during labor; 5-minute Apgar score; fetal suffering; the use of oxygen in the delivery room; the use of intermittent mandatory ventilation and the need for resuscitation measures.
Finally, the third group of variables referred to the newborn (proximal level)
Gestational age at birth (Ballard score); gender; birth weight; blood type of the newborn infant (ABO, Rh factor, phenotype); direct Coombs test; anemia; congestive heart failure; clinical complications; jaundice; hematocrit at birth and 24 h and 40 h after birth; total serum bilirubin level in umbilical cord blood; 6 h, 12 h and 40 h total serum bilirubin level after birth; peak serum bilirubin level at hospitalization; platelet count; type and duration of phototherapy; need for and number of postpartum transfusions; total duration of hospital stay (days) and number of days with negative DAT.
Initially, for the purpose of statistical analysis, exploratory data analysis was performed to assess the consistency of the data and the distribution of the variables of interest. Poisson regression models with robust variance were applied to determine the factors associated with the incidence of exchange transfusion. The factors associated with the incidence of the clinical outcome were evaluated in groups according to the nature of the independent variables of interest. The study groups were then subdivided into variables related to maternal history and prenatal care, delivery and newborn (Figure 1). The correlation matrix between the explanatory variables was analyzed to assess the possibility of multicollinearity. In each group, univariate analyses were performed, and the variables with p-values below 0.20 were included in the multivariate model. Finally, the factors that were associated with exchange transfusion at a significance level of 10% in each study group were included in the final multivariate model. The choice of factors to be included in the multivariate model was not based purely on statistical associations. A decision on which factors to include was based on a conceptual framework describing the hierarchical relationships between risk factors. At each level of final multivariate model, variables were controlled by those of same level and above, but not for inferior level. Hence the estimate obtained for each level is related to the effect of the variable adjusted by possible confounding factors, but not to possible mediating variables.
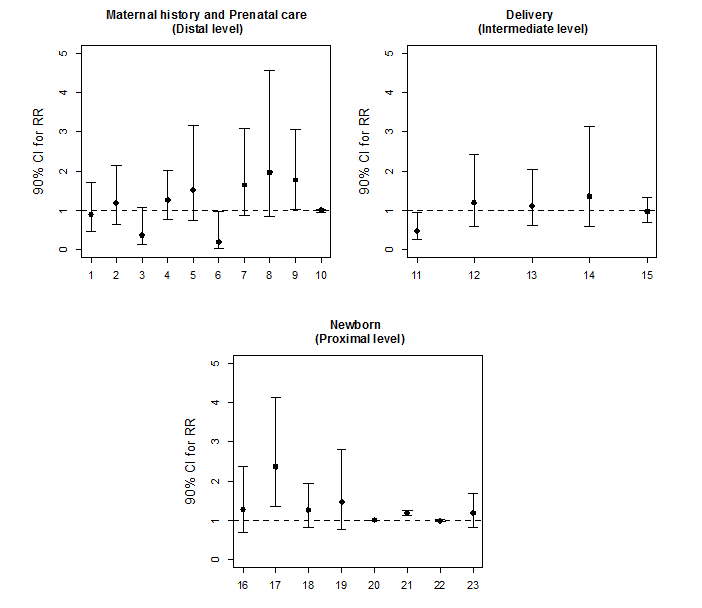
The magnitude of the association between the risk (or protective) factors and the incidence of exchange transfusion were quantified in terms of their relative risk (RR) and respective 90% confidence intervals. The software used for data analysis was SPSS Version 20.
A total of 124 pairs of mother/newborn infant medical records were analyzed according to the previously determined criteria regarding the births of fetuses with HDFN. The mean age of the maternal population was 30.8±5.9 years. The median number of pregnancies, births and abortions among the studied patients was three, two and zero, respectively. The mean gestational age calculated at birth was 35.9±2.3 weeks; newborns born by cesarean section had a lower birth weight mean value than newborns born by vaginal delivery (35.7±2.1 and 36.3±2.6 weeks, respectively), but the difference was not statistically significant. The gender distribution of the newborns was similar. The variable stillbirth comprised one single case.
In terms of maternal history and prenatal care (distal level), there was a trend for an association among the need for exchange transfusion with maternal blood type B (RR: 0.18; p=0.09) and need for intrauterine transfusion therapy (RR: 1.77; p = 0.09) (Figure 1). In the multivariate model for this level, only the need for intrauterine transfusion therapy remained a risk factor for the outcome (RR: 2.54; p=0.00) (Table 2). In the analysis of birth-related factors (intermediate level), there was a trend for an association between exchange transfusion risk and the type of delivery (vaginal or cesarean section). Vaginal delivery was defined as a protective factor (RR: 0.47; p=0.07) (Figure 1). However in the multivariate model it lost statistical power of association (Table 2). Finally, the newborn variables (proximal level) associated with exchange transfusion outcome were jaundice (RR: 2.36; p=0.01) and peak serum bilirubin level at hospitalization (RR: 1.17; p=0.00) (Figure 1). After multivariate analysis, these variables were still significantly correlated with the outcome of interest (Table 2). In the hierarchical model (Table 2), the variables that displayed a significant association with the neonatal outcome of interest were the need for intrauterine transfusion therapy, jaundice and peak serum bilirubin level.
Levels / Variables |
RR (90% CI) |
P value |
|---|---|---|
Maternal history and Prenatal care (Distal level) |
||
Maternal blood type |
|
|
Type A |
1,23 (0,75- 2,01) |
0,49 |
Type AB |
1,55 (0,68- 3,54) |
0,38 |
Type B |
0,19 (0,04- 1,03) |
0,11 |
Type O |
1,00 |
- |
IUT |
|
|
Yes |
2,54 (1,61-4,00) |
0,00 |
No |
1,00 |
- |
Delivery (Intermediate level)* |
||
Vaginal delivery |
|
|
Yes |
0,59 (0,29-1,20) |
0,22 |
No |
1,00 |
- |
Newborn (Proximal level)** |
||
Jaundice |
|
|
Yes |
2,77 (1,76- 4,39) |
0,00 |
No |
1,00 |
- |
Peak BL |
1,16 (1,11- 1,22) |
0,00 |
|
|
|
Table 2 Final hierarchical model with the variables that displayed a significant association with the need for exchange transfusion therapy
*Adjusted for variables in distal level.
**Adjusted for variables in distal and intermediate levels.
Hemolytic disease of the fetus and newborn is an avoidable cause of death in infants younger than five years of age.8 However, its preventability depends on available technology, which should be accessible to the majority of the population and which should be provided in Brazil by the country’s National Unified Heath System. In this case, the incidence of hemolytic disease of the fetus and newborn can be reduced through preventive vaccination and/or the provision of adequate care to the women and the newborns in the pregnancy-childbirth cycle.
The evaluation of the variables regarding maternal variables and prenatal care (distal level) in the final model revealed that intrauterine transfusion therapy was the only factor that represented an increased risk for a severe neonatal outcome, defined as exchange transfusion. Van Kamp et al.,9 reported that since the introduction of intrauterine transfusion therapy techniques, they have been widely used as an efficient and safe antenatal treatment of severe fetal anemia. Hence, performing intrauterine transfusion therapy during prenatal care implies that the fetus is severely afflicted with the disease and at risk of needing neonatal intervention to reduce morbidity. The current method for fetal anemia monitoring and intrauterine transfusion therapy referral is the middle cerebral artery peak systolic velocity measurement established by Mari et al.10 This method allows the identification of a fetus at risk of moderate or severe anemia when levels reach values above 1.5 multiples of the median (MoM) for a particular gestational age. The method has a sensitivity of 100% and a 12% rate of false positives. Thus, immature fetuses are subjected to intrauterine transfusion therapy to treat moderate or severe anemia and avoid the development of such conditions as hydrops and intrauterine death.11 Illanes & Soothill12 further argue that antenatal therapies can modify but are not able to fully revert the hemolytic process, determining that surveillance and neonatal care are necessary measures for an adequate outcome during follow-up. When studying the early outcome of infants subjected to intrauterine transfusion therapy, McGlone et al.,13 observed that exchange transfusion was necessary in 20% of newborns. In a study of a cases series of infants with HDFN and no other associated comorbidities, De Bouer et al.,14 attempted to establish the differences between afflicted newborns who had received intrauterine transfusion therapy and those who had not. The authors concluded that infants subjected to intrauterine transfusion therapy remain in the hospital fewer days and require fewer interventions during the neonatal period, especially regarding the need for phototherapy; however, there was no statistically significant association between the need for exchange transfusion and intrauterine transfusion therapy. This finding differs from the results of the present study, meaning that the inclusion of fetuses with a more severe condition in the study population can result in a significant association between the use of intrauterine transfusion therapy and the exchange transfusion outcome, which can become an indicator of risk in this situation. Other authors15–17 have already shown the impact of the fetus’s condition at the beginning of intrauterine therapy as a determining factor for neonatal outcome, making comparisons between the results obtained at different referral centers more difficult. The maternal blood type B regarding need for exchange transfusion was not confirmed as an independent risk factor after applying the multivariate model. The relationship of ABO incompatibility between mother and fetus as a protective factor against RhD HDFN has been established;18–20 however, there is no evidence that can explain the trend of a negative relationship of maternal type B with HDFN.
The gestational age at birth and the type of delivery of the pregnancies afflicted with HDFN have changed over time. More severe fetuses that underwent intrauterine transfusion therapy had scheduled cesarean section deliveries, which increased the rate of complications related to prematurity, such as hyaline membrane syndrome.11 Currently, the trend in the management of these fetuses is to keep them in a serial intrauterine transfusion therapy treatment plan up to the 35th week, with a delivery planned for three weeks later. This management plan has had good results with respect to neonatal outcome and had even reduced the need for exchange transfusion.21 With respect to the type of delivery, there is no consensus about whether vaginal or cesarean section is the ideal type of delivery.22 The results obtained for the variables regarding delivery exhibited a significant association between the type of delivery and a severe outcome, and vaginal delivery was a protective factor for the development of the newborn. Of note, we observed a higher mean gestational age for pregnancies that ended with a vaginal delivery compared with cesarean sections. The tendency to choose a cesarean section for more severe cases of HDFN was observed by Lobato & Soncini17 in a previous study from the same unit.
Neonatal hyperbilirubinemia is treated by a referral to exchange transfusion therapy. Hence, in terms of newborn variables, a strong association between infants who exhibited higher total serum bilirubin levels and a diagnosis of jaundice after birth was expected. Another important resource is phototherapy. This method reduces serum bilirubin levels by photooxidation and by converting bilirubin into water-soluble substances, a process termed photoisomerization. The efficacy of this therapy depends on several factors, including the spectral quality of the emitted light, the irradiance, the exposed area of the newborn and the total serum bilirubin level at the beginning of the exposure.15 The protocol followed by the Institute’s neonatology team during the study period dictated that all infants afflicted with HDFN be referred to phototherapy at an early stage, immediately after birth.23 Therefore, the use of phototherapy was not included as a variable related to exchange transfusion. It was only of interest for establishing the relationship between the duration of phototherapy and severe outcomes and the impact of this relationship on the total duration of hospital stay. We observed that infants who were not subjected to exchange transfusion spent more time in phototherapy treatment and exhibited longer total hospital stays. In a previous evaluation of patient flow in our Institute, Sá et al.,24 observed that 50 patients/year exhibited Rh alloimmunization at the first medical appointment. Among them, 25% of the pregnant women or their fetuses required some type of invasive intervention (intrauterine transfusion therapy or exchange transfusion). Furthermore, approximately 90% of the newborns had their discharge delayed because of the need for phototherapy. Another therapeutic resource used during this period was human intravenous immunoglobulin (IVIg), which has been recommended as an alternative therapy. The use of this substance is generally associated with phototherapy for the management of HDFN, with the goal of reducing the need for exchange transfusion.25,26 During the evaluation period for this cohort, a clinical study was conducted at our Institute to assess the efficacy of this type of therapy in reducing the severity of HDFN in infants. However, the study did not show that this technology had an impact on the infants’ outcomes; thus it must not constitute a confounding factor for the obtained results.23
The use of exchange transfusion as a neonatal therapy allows the removal of sensitized fetal red blood cells, circulating maternal antibodies and free bilirubin from the blood of the newborn and restores Rh-antigen-free red blood cells by substituting the newborn’s blood with that of the Rh(D)-negative donor. The introduction of this therapy, together with anti-D prophylaxis, was the factor that had the highest impact on the progression of HDFN. The improvement in prenatal care, together with the advance in available technologies for ante- and postnatal treatment, has led to an important reduction in morbidity and mortality related to maternal alloimmunization. However, the prognosis of these infants can also be severely affected by varying degrees of cerebral paralysis, deafness or a developmental deficit.27 The present study provides relevant information on the influence of prognostic factors related to the severity of HDFN in the cases that were monitored at our referral unit. The results exhibited here provide a valuable contribution to the goal of improving the care provided to this at-risk population in the Brazilian context.
None.
The authors declare there is no conflict of interests.

©2015 Moreira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.