Open Access Journal of
eISSN: 2575-9086


Research Article Volume 5 Issue 1
1Institute of Biotechnology, Federal University of Uberlândia, Campus Umuarama, 38400-902, Uberlândia, Minas Gerais, Brazil
2Institute of Exact and Natural Sciences of Pontal, Federal University of Uberlândia, Campus Pontal, 38304-402, Ituiutaba, Minas Gerais, Brazil
Correspondence: Foued S Espindola, Institute of Biotechnology, Federal University of Uberlândia, Campus Umuarama, 38400-902, Uberlândia, Minas Gerais, Brazil
Received: October 17, 2022 | Published: November 4, 2022
Citation: da Costa AV, Calábria LK, de Rezende AAA, et al. Vochysia rufa and glibenclamide promote effects on oxidative damage and in proteins implicated in vesicular trafficking in diabetic rat brain. Open Access J Sci. 2022;5(1):85-91. DOI: 10.15406/oajs.2022.05.00180
Many Vochysiaceae species are widely used in folk medicine to treat some diseases. Vochysia rufa, popularly known as “quina-doce”, has been used in folk medicine to treat type 1 and type 2 diabetes mellitus in the state of Minas Gerais, Brazil. Although the antidiabetic and antioxidant effects and phytochemical profile of Vochysia rufa have already been elucidated, further studies are needed on the effects of this treatment in specific tissues, such as the brain. This study investigated the effect of aqueous extract of Vochysia rufa in diabetic rat brains and for this purpose, oxidative stress markers and the expression/localization of proteins implicated in vesicular trafficking were evaluated. Thirty-two rats were randomized into four groups (non-diabetic, diabetic non-treated, diabetic treated for 43 days with glibenclamide - 6 mg/kg or Vochysia rufa - 500 mg/kg). The extract was administered by gavage for 43 days. Analyses were conducted of enzymes concentration and activity in the brain. The protein levels and localization of myosin-Va, CaMKII, synapsin, SNAP-25 and GLUT4 were also analyzed. Vochysia rufa extract decreased superoxide dismutase, glutathione peroxidase, reduced glutathione, total sulfhydryl and lipid peroxidation levels and increased glutathione S-transferase levels. Additionally, Vochysia rufa treatment increased the expression of myosin-Va, CaMKII and also synapsin, which were confirmed by immunolocalization. The treatment with aqueous extract of Vochysia rufa reduces oxidative stress on diabetic rat and protecting the brain from damage caused by hyperglycemia.
Keywords: diabetes mellitus, oxidative stress, myosin-Va, synapsin, CaMKII
Diabetes is characterized by persistent hyperglycemia, which leads to increasing reactive oxygen species and consequent changes tissue damage.1–3 There is an increasing appreciation that memory and learning impairment,4,5 as well as morphological changes, such as decreased nuclear diameter of pyramidal neurons of the hippocampus is related with diabetes.6 Hyperglycemia leads to imbalance in the oxidative status of the neural tissue, increasing free radical production via auto-oxidation of glucose, glycation of proteins and low production of antioxidant defenses, favoring an oxidative stress situation,1 could be controlled by antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.7 Hyperglycemia alters cellular redox balance and protein trafficking involved in synaptic vesicle docking. This may be related due to changes in expression of myosins,6,8,9 GLUT4,10 pre synaptic proteins, such as synapsin-1, synaptophysin and SNAP-25.11 Decreased CaMKII phosphorylation in the hippocampus, which may be associated with cognitive dysfunction, is also observed.12 Many plants have confirmed hypoglycemic properties13 and several studies appointed that plant extracts are hypoglycemic agents and alleviates oxidative stress in diabetic rats14,15 such as Pouteria ramiflora,6 which reduced the oxidative damage in brain tissue; Teucrium polium,16 ameliorate memory impairments and brain tissue oxidative damage and Vochysia rufa, that was able to control glycemic levels and reduce oxidative stress in liver.17
Thus, the present study was designed to evaluate the effect of Vochysia rufa treatment on oxidative stress and expression of proteins implicated in vesicular trafficking such as myosin-Va, CaMKII, synapsin-1, SNAP-25, GLUT4 and the distribution of these proteins in the cortex and hippocampal regions of the diabetic rat brain.
Plant material and aqueous extract
Stem barks from Vochysia rufa Mart. were collected from the Cerrado biome in the outskirts of Abadia dos Dourados/MG, Brazil, (latitude 18º 27` 50.5’’ and longitude 47º 23` 37.2’’) from October, 2010 to February 2011. The plant was botanically identified and a voucher specimen deposited (number 58,888) at the Herbarium Uberlandensis of the Biology Institute at Federal University of Uberlandia, Uberlândia/MG, Brazil. Then, using a stove at 40°C, the stem barks were dried and powdered. Through maceration of 200 g of powdered bark in 1 L of distilled water for 24 h (1:5 w/v) at room temperature, the aqueous extract was obtained. The extract was filtered and centrifuged at 2000 xg at 4°C, for 15 min. Thus, the supernatant was collected, frozen, lyophilized and stored at -20°C. The phytochemical characterization of the Vochysia rufa extract and its molecular identification by HPLCESI-MS/MS were revealed by Gouveia et al.18 and Franco et al.19 This study received authorization for access and remittance of genetic material for scientific research by SISGEN (n° A933BED).
Animals
Male Wistar rats (approximately 8 weeks and 200-220 g) were housed under standard conditions (22 ± 1 °C, humidity 60 ± 5 %, and 12 h light/12 h dark cycle) with food (65.82% carbohydrate, 5.36% fiber, 21.0% protein and 4.96% fat; Bio base, Brazil) and water ad libitum. Procedures for animal handling and use followed the resolutions proposed by the Brazilian Society of Laboratory Animal Science and the Ethics Committee for Animal Research of the Federal University of Uberlandia, Uberlândia/MG, Brazil (CEUA/UFU 060/10).
Induction of diabetes mellitus
The rats were fasted for 24 h, anesthetized by intraperitoneal injection of xylazine (10 mg/kg body weight, bw)/ketamine (75 mg/kg bw) and streptozotocin (40 mg/kg bw, 0.01 M citrate buffer, pH 4.5; Sigma-Aldrich, USA) via the penile vein (2 mL/kg bw) was injected.6,8,9,20,21 Non-diabetic rats received equal volume of citrate buffer through penile vein. Then animals were fasted for another 90 min after injection. Ten days after the streptozotocin injection, rats with fasting blood glucose levels above 250 mg/dL were scored as diabetic. Glucose levels were monitored throughout the experiment Biocheck Strips Glucose Test (Bioeasy, Brazil).
Groups and treatments
The rats were randomly divided into four groups (n=8 rats/group): non-diabetic (ND), diabetic (D), diabetic treated with glibenclamide (6 mg/kg bw orally; (DG; Biosintética Farmacêutica Ltda, Brazil), and diabetic treated with Vochysia rufa extract (500 mg/kg bw orally; DV). The rats received water and treatment administered by gavage for 43 days. Body weight was recorded weekly. After this period, the animals were fasted for 12 h, anesthetized with xylazine (10 mg/kg body weight, bw)/ketamine (75 mg/kg bw), followed by cervical dislocation. Afterwards, brains were surgically removed and immersed in liquid nitrogen (n=4 rats/group) or 10% formaldehyde solution (n = 4 rats/group).
Biochemical analysis
Each brain was homogenized separately on ice in homogenization buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 2 mM EGTA, 2 mM dithiothreitol, 1 mM benzamidine, 0.5 mM phenylmethanesulfonyl-fluoride, 0.1 M aprotinin, 0.1 mM pefabloc). The homogenates were centrifuged at 15,000 × g for 30 min at 4 °C, and the total protein concentration in the supernatants was measured by the Bradford assay.22
Oxidative stress biomarkers
Superoxide dismutase (SOD) activity was determined by adenocromo inhibition during adrenaline oxidation. The results were expressed as U/μg of protein.23 Glutathione peroxidase (GPx) activity was quantified by the oxidation of NADPH in the presence of glutathione reductase (GR), measured at 340 nm and expressed in mmol.min-1.g-1.24 Glutathione S-transferase (GST) activity was measured at 340 nm using CDNB as substrate and a 0.15 M reduced glutathione (GSH).25 The levels of GSH were determined using DTNB (2-dithionitrobenzoic acid)26 and the level of sulfhydryl was determined using 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), according to the method of Faure and Lafond.27 The results were expressed in μmol/mg of protein. Also, the level of malonyldialdehyde (MDA), as a substance that reacts with thiobarbituric acid were estimated colorimetrically.28 The results were expressed in nmol.g-1 tissue.
Antibodies
Rabbit affinity-purified polyclonal antibodies included: anti-chicken brain myosin-Va (anti-myosin-Va) head domain recombinant protein29–31 and anti-glucose transporter GLUT4 (anti-GLUT4) by Abcam Inc. (Cambridge, USA). Mouse monoclonal antibodies used: anti-calcium/calmodulin-dependent protein kinase IIα (anti-αCaMKII), anti-synaptosomal-associated protein-25 (anti-SNAP-25), and anti-synapsin by BD Transduction (San Jose, USA) and anti-b-actin by Sigma-Aldrich (St. Louis, USA). Secondary antibodies (anti-mouse IgG and anti-rabbit IgG) conjugated to peroxidase were obtained from GE Healthcare (Buckinghamshire, UK).
Western blotting
Aliquots of supernatant samples (see Biochemical analysis) containing 30 µg of protein were solubilized in a small volume of electrophoresis sample buffer,32 analyzed by SDS-PAGE on 5-22% polyacrylamide gradients, and electroblotted onto nitrocellulose membranes in Tris-glycine buffer. The membranes were probed with 0.2 µg/mL primary antibodies. β-actin antibody was used as a control. The membranes were incubated with peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG diluted 1:2,000. Antibodies bound to the membranes were visualized by chemiluminescence after incubation with ECLTM followed by exposure to HyperfilmTM (GE Healthcare, Sweden), according to the manufacture’s instructions. The intensity of the protein bands was analyzed and compared using Scion Image software, version Alpha 4.0.3.2 (Scion Corporation, USA), and the results were expressed as percentage of total protein content.
Immunohistochemistry
The brains were fixed with 10% formaldehyde solution in 0.1 M phosphate-buffered saline (pH 7.4) for 24 h, dehydrated in ethanol, rinsed in xylene and embedded in paraffin.6,9,21 Seven-micrometer sections were pretreated in microwave for 5 min with 4 mM citrate buffer (pH 6.0) containing 0.025% Tween 20. The sections were then incubated with primary antibodies for 16 h followed by incubation with a Post Primary Block NovoLink™ Max Polymer Detection System (Novocastra Laboratories Ltd., UK). The sections were incubated with the NovoLink polymer for 30 min at 37 °C. Chromogen was developed using 3,3′-diaminobenzidine, and then the material was counterstained with hematoxylin, dehydrated, mounted with ERV-Mount (EasyPath, Brazil) and analyzed with the 40× objective lens of Leica DM500 microscope (Microsystems, Germany). Negative controls were prepared by omitting the primary antibody from the reaction.
Statistical analysis
All values obtained were expressed as mean ± SEM (standard error mean). Statistical analysis was performed with the Student t-test, and data were analyzed using SigmaStat 3.5 software (Systat Software Inc., USA). A p-value < 0.05 was considered significant.
It has been reported that deleterious effects of reactive oxygen species (ROS)-mediated injury are frequently observed in diabetic patients and animal models.4 The deleterious effects are related to cellular proteins disruption, lipid membranes oxidation, nucleic acids damage, and cell death.1 The central nervous system is especially vulnerable to oxidative damage.33 In the present study, oxidative stress markers and antioxidant enzyme levels were evaluated in diabetic rats treated with glibenclamide and an aqueous extract of Vochysia rufa. Figure 1 depicts SOD, GPx and GST enzymes activities, GSH enzyme concentration, total sulfhydryl and MDA in the brain. GPx and GST activities decreased in the diabetic when compared to non-diabetic rats, meanwhile SOD, GSH, total sulfhydryl and MDA levels increased. These results are attributable to a high rate of oxygen consumption and high polyunsaturated fatty acid content especially pronounced in diabetic brains,34 once hyperglycemia status reduces antioxidant levels and increases free radicals production with subsequent activation of redox-sensitive genes.35,36
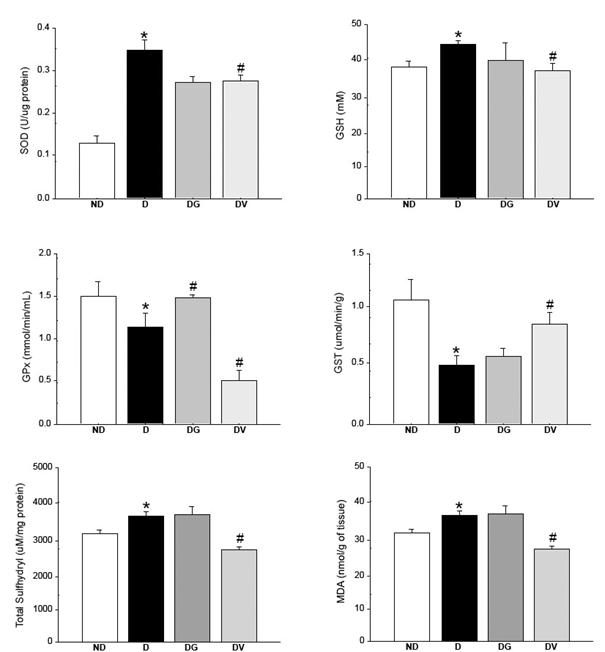
Figure 1 Effect of treatment with Vochysia rufa extract on superoxide dismutase (SOD), reduced glutathione (GSH), glutathione peroxidase (GPx) and glutathione S-transferase (GST) activity, total sulfhydryl and malondialdehyde (MDA) concentrations in the brain of streptozotocin-induced diabetic Wistar rats. ND: non-diabetic, D: diabetic, DG: diabetic with glibenclamide, DV: diabetic with Vochysia rufa. Data are reported as mean ± SEM (n = 4 rats/group). *compared to non-diabetic, # compared to diabetic.
It is believed that plants with antioxidant properties can prevent or protect tissues against the injuries of free radicals.37 Treatment with Vochysia rufa aqueous extract significantly decreased SOD, GSH and GPx activities, total sulfhydryl and MDA levels. Moreover, the group treated with the plant extract ameliorated GST activity. The gender Vochysia has abundant polyphenols and triterpenes38 with antidiabetic properties,39 but the total polyphenols, flavonoids and condensed tannins content were only recently revealed for Vochysia rufa ethanolic extract of stem bark.19
Moraes et al.17 and Gouveia et al.18 using the same aqueous extract of the present study concluded that hepatic and pancreatic oxidative stress, respectively, caused by streptozotocin-induced diabetes, reduced after treatment. Moreover, Gouveia et al.40 revealed partial composition of Vochysia rufa stem bark and its effect against oxidative damage in endothelial cells, and in the Vochysia rufa extract was found to be composed of free sugars (inositol, galactose, glucose, mannose, sucrose, arabinose, and ribose) as the main metabolites.39 We observed the same protective effect in the rat brain.
Several studies have shown that plant extracts can reverse changes in the rat brain caused by diabetes. For instance, a curcumin supplement decreases lipid peroxidation levels,41 Bacopa monnieri modulates SOD and GPx activities in different brain regions42 and Pouteria ramiflora increases GPx and SOD levels and also decreases lipid peroxidation.6 On the other hand, when used as a positive control glibenclamide increased GPx and decreased SOD activities, when compared to diabetic groups (Figure 1). This sulfonylurea affects beta cells stimulating insulin secretion,43,44 which probably reduces oxidative stress diminishing the hyperglycemic status.
Diabetes is related to changes in neurotransmission that may cause cognitive dysfunction.5 Neurotransmission only occurs after formation of a complex, evolving proteins of the synaptic vesicle and the plasma membrane. However, if one side of this pair is missing, the complex will be unstructured.45,46 Proteins such as myosin-Va, CaMKII, synapsin and SNAP-25 are involved in neurotransmission, whereas GLUT4 participates as an insulin-sensitive glucose transporter. Figure 2 shows the immunoreactivities of myosin-Va (190 kDa), α-CaMKII (52 kDa), synapsin (80 kDa), SNAP-25 (25 kDa) and GLUT4 (56 kDa) measured densitometrically. Distribution of these proteins in the frontal (Figure 3-4) and occipital (Figure 5-6) cortex and hippocampus (Figure 7) was revealed by immunohistochemistry.
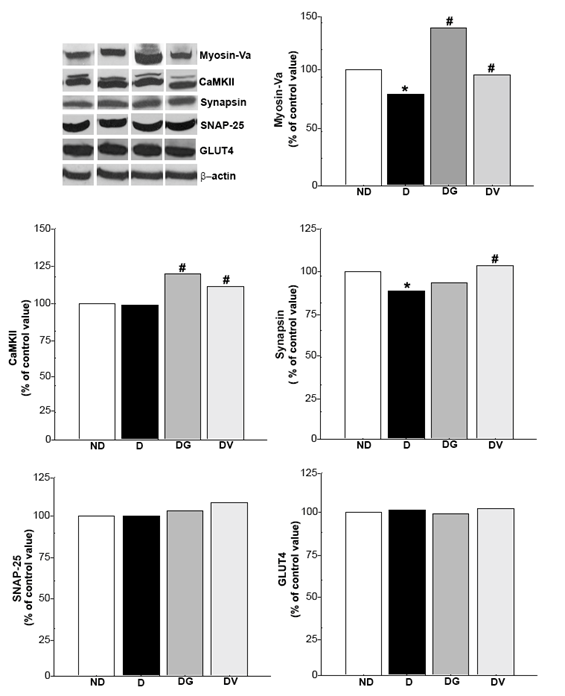
Figure 2 Effect of treatment with Vochysia rufa extract on myosin-Va, α-CaMKII, synapsin, SNAP-25 and GLUT4 protein levels in the brain of streptozotocin-induced diabetic Wistar rats. ND: non-diabetic, D: diabetic, DG: diabetic with glibenclamide, DV: diabetic with Vochysia rufa. The protein level in the immunoblot was determined by densitometric readings and data are expressed as percentage within groups (n = 4 rats/group). *compared to non-diabetic, # compared to diabetic.
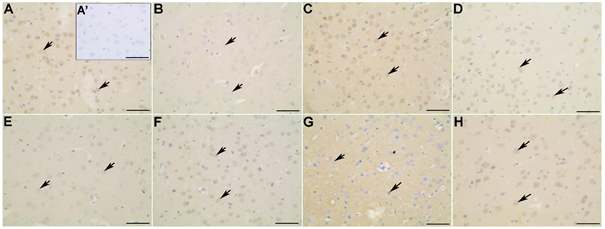
Figure 3 Myosin-Va (A-D) and α-CaMKII (E-H) distribution in the frontal cortex of non-diabetic (A; A’; E), diabetic (B; F), diabetic treated with glibenclamide (C; G), and diabetic treated with Vochysia rufa extract (D; H) rat brains. Pyramidal neurons (arrow). Negative control (A’). Bar: 25 μm (A-H) and 50 μm (A’).
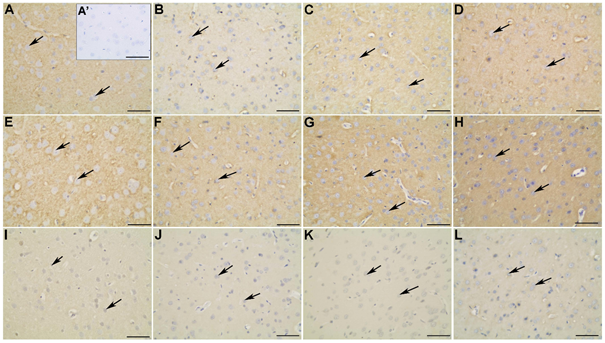
Figure 4 Synapsin-1 (A-D), SNAP-25 (E-H) and GLUT4 (I-L) distribution in the frontal cortex of non-diabetic (A; A’; E; I), diabetic (B; F; J), diabetic treated with glibenclamide (C; G; K), and diabetic treated with Vochysia rufa extract (D; H; L) rat brains. Pyramidal neurons (arrow). Negative control (A’). Bar: 25 μm (A-L) and 50 μm (A’).
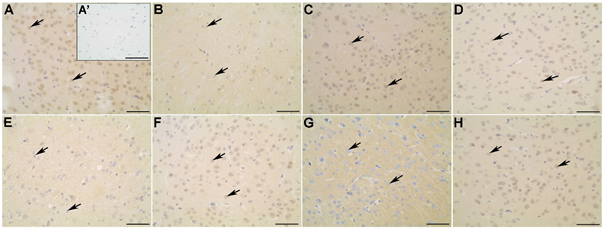
Figure 5 Myosin-Va (A-D) and α-CaMKII (E-H) distribution in the occipital cortex of non-diabetic (A; A’; E), diabetic (B; F), diabetic treated with glibenclamide (C; G), and diabetic treated with Vochysia rufa extract(D; H) rat brains. Pyramidal neurons (arrow). Negative control (A’). Bar: 25 μm (A-H), and 50 μm (A’).
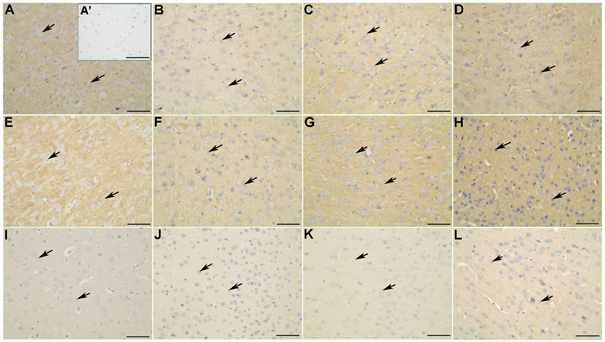
Figure 6 Synapsin-1 (A-D), SNAP 25 (E-H) and GLUT4 (I-L) distribution in the occipital cortex of the non-diabetic (A; A’; E; I), diabetic (B; F; J), diabetic treated with glibenclamide (C; G; K), and diabetic treated with Vochysia rufa extract (D; H; L) rat brains. Pyramidal neurons (arrow). Negative control (A’). Bar: 25 μm (A-L), and 50 μm (A’).
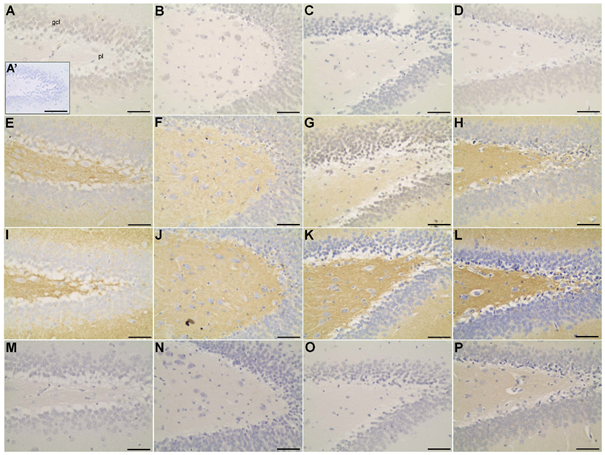
Figure 7 Immunolocalization of α-CaMKII (A-D), synapsin-1 (E-H), SNAP 25 (I-L) and GLUT4 (M-P) in dentate gyrus of the hippocampus of non-diabetic (A; A’; E; I; M), diabetic (B; F; J; N), diabetic treated with glibenclamide(C; G; K; O), and diabetic treated with Vochysia rufa extract (D; H; L; P) rat brains. Neurons of the granular layer (gcl); neurons of the polymorphic layer (pl). Negative control (A’). Bar: 25 μm (A-P), and 50 μm (A’).
Western blot analysis displayed that diabetic rats had decreased levels of myosin-Va in the brain when compared to the non-diabetic group. Low levels of this protein was also observed in diabetic rats by da Costa et al.,6,9 but treatment with either Vochysia rufa or glibenclamide reversed this effect (Figure 2). Glibenclamide also reverse damage caused by diabetes on myosin-IIB expression and reduce the patho-physiological changes in the brain.20 Myosin-Va distribution in non-diabetic is more intense in the cytoplasmic soma and extension of pyramidal neurons with intense perinuclear labeling in both cortex (Figure 3A-D; Figure 5A-D), a similar pattern was described by Takagishi et al.47. Myosin-Va is a molecular motor associated with transport vesicles in neurons48 and its expression changed after treatment with aqueous Pouteria ramiflora extract, likely as a response to the plant’s antioxidant and neuroprotective properties.6 Similarly, it is possible that the antioxidant properties of the Vochysia genus had the same effect.49
Protein levels of α-CaMKII were kept the same, comparing non-diabetic and diabetic rats, but increased when treated with glibenclamide or Vochysia rufa extract (Figure 2). α-CaMKII distribution was uniform in non-diabetic and diabetic rat brains, and produced less intense marking in the cytoplasm soma and perinuclear region in frontal cortex (Figure 3 E-H). The nucleus of neurons were stained more in rats treated with glibenclamide than in non-diabetic, diabetic and diabetic treated with Vochysia rufa extract in occipital cortex (Figure 5E-H). CaMKII is an important enzyme in the regulation of glutamatergic synapses50 and is essential for synthesis of insulin granules, modulation of cytoplasmic content of ATP, and activation of synapsin.51 Thus, it is possible that hyperglycemia activates CaMKII and alter phosphorylation of its multiple targets to exert both acute and chronic effects in many tissues,52 and treatment with plant extract or antidiabetic stimulate insulin release by CaMKII action.
Although streptozotocin-induced diabetes cause prominent changes in neuronal calcium homeostasis53 as well as an increase CaMKII of total amount, was observed in central nervous system and in different tissues of diabetes types 1 and 2.12,54 Those alterations were not observed in our study, and the activation mechanism for this disease has not been elucidated yet. Other important point is that the oxidative stress potentiates synaptic transmission via activation of CaMKII in several pathophysiological conditions,55,56 including diabetes. Synapsin I is a substrate for CaMKII in the pancreas, but this event appears not to be important for the mobilization of insulin granules.57 Diabetic rats have decreased synapsin in the brain when compared to the non-diabetic group, whereas treatment with Vochysia rufa extract increased the protein levels (Figure 2). Plants are constantly used to improve hyperglycemia and thereby oxidative stress.15,58,59
Moreover, synapsin distribution in the frontal (Figure 4A-D) and occipital (Figure 6A-D) cortex of non-diabetic rat brains was intense in the soma and extension of pyramidal neurons compared to diabetic, and in the cytoplasm of neurons of the granular layer and polymorphic layer in hippocampus (Figure 7E-H). Immunohistochemical staining demonstrated an intense immunolabeling of synapsin I in hippocampal pyramidal neurons in type 1 diabetic rats.12 This protein is involved in the regulation of exocytotic release vesicles.60 Our blotting analyses showed no differences in SNAP-25 and GLUT4 expression between groups (Figure 2) even in brain distribution. SNAP-25 participates in SNARE complex assembly61,62 and the glucose pulse paradigm induced its expression.63 SNAP-25 was intense immunolabeling in the frontal (Figure 4E-H) and occipital (Figure 6E-H) cortex of the non-diabetics and diabetics, predominantly in the soma and extension of pyramidal neurons, and in the cytoplasm of neurons of the granular layer and polymorphic layer in hippocampus (Figure 7I-L). GLUT4 distribution in the frontal (Figure 4 I-L) and occipital (Figure 6 I-L) cortex does not differ among the groups, with weak and diffuse label in the cytoplasm and fibers, including hippocampus (Figure 7 M-P). There is no difference in the expression of glut4 mRNA in the frontal cortex64 and expression of GLUT4 protein in the cerebral cortex of streptozotocin-diabetic rats10 even with increased glucose levels.
Oxidative stress associated with the expression of protein vesicles that may affect synaptic function and plasticity or alter the release of vesicles, appears to be a presynaptic deleterious mode of action of reactive oxygen species. We suggest that, by decreasing oxidative stress, Vochysia rufa extract and glibenclamide improved the proteins levels associated with vesicle trafficking and anchoring. Uncontrolled diabetes markedly alters hippocampal genes expression, particularly for genes thought to be involved in synaptic function, plasticity and for neurogenesis. Plant extracts had no effect on the levels of binding protein for complex formation, but high concentration of polyphenols could decreases the interaction between vesicle proteins. It was confirmed that Vochysia rufa extract increase expression levels of the myosin-Va, CaMKII and synapsin, and reduces oxidative stress, thus, protecting the brain from damage caused by hyperglycemia. Future studies are need to understand the mechanism of action of Vochysia rufa in brain of diabetics.
The authors would like recognize Aline Borges Rodovalho, Camilla Manzan Martins, Douglas Carvalho Caixeta, Hélen Lara Machado and Izabela Barbosa Moraes (Federal University of Uberlândia) for contributions to experimental procedures.
AVC and LKC performed the acquisition and interpretation of data, and were involved in drafting the manuscript. AAAR was involved in the interpretation of data and critical revision of the manuscript for important intellectual content. FSE conceived and designed the study. All authors reviewed and approved of the final manuscript.
This research was funded by FAPEMIG (APQ-01271-10 and APQ-01178-11) and CNPq (308965/2015-9).
The authors have declared that no competing interests exist.

©2022 da, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.