Open Access Journal of
eISSN: 2575-9086


Review Article Volume 7 Issue 1
1Microbiology and Food Quality Control Laboratory (LAMICODA), Higher School of Biological and Food Techniques (ESTBA), University of Lomé, Lomé, Togo
2Laboratory of Biomedical Sciences, Food and Environmental Health - Research Unit in Biomedical Sciences and Bioactive Substances (LaSBASE-UR-2SB), Higher School of Biological and Food Techniques (ESTBA), University of Lomé, Lomé, Togo
3Department of Biochemistry /Nutrition, Laboratory of Biochemistry applied to Nutrition, Faculty of Sciences, University of Lomé, Lomé, Togo
4Laboratory of Biology, Phytochemistry, Toxicology, Pharmacology and Agrifood (BioPhytToPharmA), Institute African Biomedical, Agrifood, Societal and Environmental Sciences (IASBASE), Lomé-Togo
5National Malaria Control Program (PNLP), Ministry of Health, Public Hygiene and Universal Access to Care, Lomé, Togo
Correspondence: Gbekley EH, Microbiology and Food Quality Control Laboratory (LAMICODA), Higher School of Biological and Food Techniques (ESTBA), University of Lomé, Lomé, Togo, Tel 91307841
Received: February 10, 2024 | Published: February 27, 2024
Citation: Hoinsou Y, Koula FC, Gbati L, et al. Synthesis of current data on typhoid fever. Open Access J Sci. 2024;7(1):52-59. DOI: 10.15406/oajs.2024.07.00214
Typhoid fever is an infectious disease that affects millions of people around the world. It is most often encountered in developing countries where hygiene conditions are poor. Mortality is estimated between 1 and 4% in children over 5 years old and in adults. The sources of contamination are varied with several phases contributing to the penetration of the responsible germ into the human body. For adequate management of the infection, the medical biology laboratory remains essential for the isolation and identification of the germ responsible for the disease through increasingly specific examinations. This work aims to make a bibliographical synthesis on the responsible germ, its physiopathology as well as the different laboratory techniques for isolating the bacterium of the genus Salmonella. To do this, a literature review was carried out in scientific databases with keywords for this purpose.
Keywords: typhoid fever, epidemiology, physiopathology, medical biology, laboratory
Typhoid fever is a systemic infection that poses a threat to populations. It is a major public health concern in developing countries.1 In many developing countries, it is an endemic infection linked to precarious sanitary conditions. It is an oro-fecal-transmitted disease, favored by overcrowding, untreated water and poor sanitation.2 Most Salmonella infections are caused by the consumption of contaminated food or water.3
Until the beginning of the 20th century, typhoid fever had a worldwide distribution, including Europe and Western countries. The considerable improvement in hygiene conditions in developed countries has led to the virtual disappearance of this infection, which is now mainly an imported disease. Today, typhoid fever is mainly found in Asia, Africa and Latin America.4
Each year, typhoid fever affects between 11 and 20 million people, resulting in 128,000 to 161,000 deaths.5 To this day, typhoid fever continues to pose a number of problems, partly due to the failure to eradicate the bacteria, and partly due to the ever-increasing resistance to antibiotics. Effective treatment therefore requires knowledge of the biology of the disease and mastery of the tests needed to isolate and characterize the causative germ, as well as the implementation of systems to monitor and prevent infection, hence the importance of medical biology.
Medical biology is a medical specialty whose aim is to carry out examinations to measure the various constituents of biological fluids (blood, urine, cerebrospinal fluid, etc.), to interpret them in relation to the patient's condition and to pass on conclusions to the clinician.6 The medical biology examination is therefore a medical act which contributes to prevention, screening, diagnosis or risk assessment of pathological conditions, and to therapeutic decision-making and management.6,7 The medical biology laboratory (LBM) is a place where medical biology examinations are carried out. It is designed to carry out haematological, microbiological, immunoserological, parasitological and mycological analyses.8 The aim of this paper is to summarize the literature on the germ responsible for typhoid fever, its pathophysiology and the role of the laboratory in managing the disease.
A systematic search was carried out in the Pub Med and google scholar databases using the following keywords Typhoid fever, Epidemiology, Physiopathology, laboratory, medical biology, typhoid fever, for articles and selected references of these articles. The 58 selected articles deal with epidemiological studies of typhoid fever, the prevalence of the disease worldwide, the medical biology laboratory, technological approaches to eradicating the disease, etc.
Case definition
Definitions are those of the WHO Typhoid Fever Surveillance System.9
Confirmed case
laboratory confirmation by culture or molecular methods of Salmonella typhi or detection of S. typhi DNA from a normally sterile site.
Suspected case
Fever for at least three of the last seven days in a person residing in an endemic area, or following travel from an endemic area within the last twenty-eight days, or within 28 days of family contact with a confirmed case of typhoid fever.
Indeterminate or doubtful case
Fever lasting at least three of the last seven days, with or without additional clinical features observed during the particular epidemic.
Epidemiology
Contagiousness and incubation period
The inoculum required to cause infection is of the order of 106 CFU per ml. However, a low inoculum 103 can cause symptoms if the host's intestine is not in physiological conditions (pH between 6 and 8). Incubation of the disease (time between bacterial entry into the body and onset of symptoms) is 7 to 21 days.10,11 The length of incubation, the onset and severity of symptoms depend on the quantity of inoculum, the host's condition (immunosuppression or a weakened immune system due to past or present infection) and, above all, the virulence of the strain coming into contact with the organism. During incubation, in some people, a fleeting diarrhea qualified as an episode of gastroenteritis is observed 12 to 48 hours after ingestion of the germ.11
Demographics
Today, typhoid fever is mainly found in Asia, Africa and Latin America.4 Each year, typhoid fever affects between 11 and 20 million people, resulting in 128,000 to 161,000 deaths Figure 1.5
Lethality
Mortality is estimated at between 1% and 4% in children over 5 and in adults. In the absence of treatment, the mortality rate is 10-20%. In children under 4, the mortality rate is ten times higher than in children over 4.12
|
USA |
Brazil |
China |
Italy |
Spain |
France |
Egypt |
Algeria |
Morocco |
Tunisia |
|
|
Total cases |
3.901.026 |
2.100.112 |
83.682 |
244.434 |
307.335 |
174.674 |
87.775 |
23.084 |
17.236 |
1.374 |
|
Total deaths |
143.321 |
79.535 |
4.634 |
35.045 |
28.42 |
30.152 |
4.302 |
1.078 |
273 |
50 |
|
Mortality rate |
5.72 |
5.42 |
5.5 |
14.41 |
11.24 |
18.97 |
3.67 |
6.97 |
2.48 |
4.5 |
Physiopathology
Structure of the microorganism(s) involved in the infection Typhoid fever is caused by a strictly human salmonella serovar: S. typhi, Salmonella are Gram-negative bacilli (rods) belonging to the Enterobacteriaceae family. They measure 0.7 to 1.5 μm in diameter, and 2 to 5 μm in length, with a flagellum. The Salmonella genus is mostly self-motile with peritrich flagellum (with the exception of Salmonella gllinarum).13
Salmonella are aero-anaerobic, reduce nitrate to nitrite, can use citrate as their sole carbon source, ferment glucose but not lactose or sucrose, and produce gas from glucose (except Salmonella typhi).
Biochemical characteristics of the Salmonella genus
The general biochemical characteristics of most serotypes isolated from humans and warm-blooded animals are as follows:
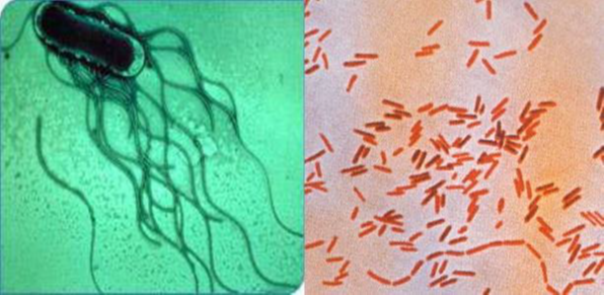
Figure 2 Salmonella morphology, Left: electron microscopy. Right: light microscopy after Gram staining.4
Transmission
Contamination can be direct, via dirty hands. This is the case for personnel who come into contact with typhoid patients, who may contaminate themselves with the excreta of the sick. Contamination is very often indirect. Although relatively fragile, typho-paratyphoid bacilli survive for some time in the external environment and spread easily.2
Entry of microorganisms into the body and multiplication
Salmonella development in the body takes place in three stages: the adhesion and invasion phase, the penetration phase and the colonization phase.12,14,15
Adhesion and invasion phase
This adhesion and invasion phase takes place in the intestinal lumen. Salmonella develop appendages called invasomes on the apical surface of intestinal epithelial cells. These appendages produce proteins that cause the epithelial cells to undulate, corresponding to degeneration of their microvilli and brush border Figure 3.
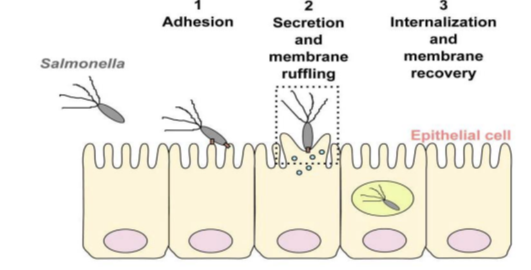
Figure 3 Salmonella adhesion and invasion of epithelial cells, and internalization into the membrane.16
The previously degenerated brush border is reformed, with changes to its cytoskeleton. Salmonella are found inside the cell in large vacuoles. Epithelial cells become phagocytic cells. Salmonella usually interact with the M cells of Peyer's patches (lymphoid follicles, the main component of digestive lymphoid tissue). These cells are capable of capturing antigens in vesicles containing proteases, and then destroying them to protect the organism against aggression. These steps do not take place in Salmonella, as they are not destroyed. Salmonella entry into these M cells is always rapidly followed by M cell destruction Figure 4.16,17
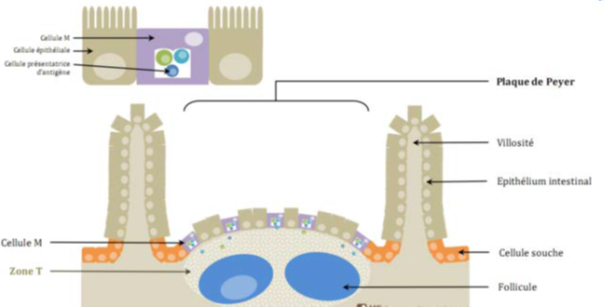
Figure 4 Tissue structure of Peyer's patches.18
Penetration phase
After a period of multiplication lasting between 12 and 24 hours, most epithelial cells contain Salmonella in vacuoles. These vacuoles are in the phagocytes of the lamina propria and submucosa. This is the epithelial penetration phase Figure 5.14
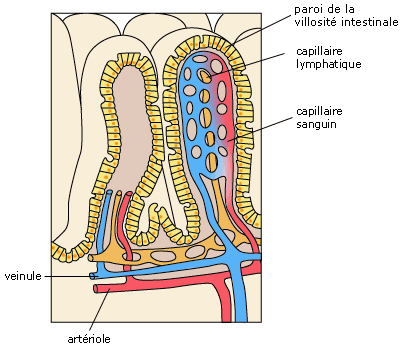
Figure 5 Representation of the intestinal wall.12
The body fights this spread by sending out phagocytes: neutrophils, eosinophils or monocytes/macrophages. However, within the phagocyte, bacteria are neither killed nor destroyed. They persist and develop within the phagocytes. They survive within the macrophages by reducing the acidity of the phagosomes and remaining in the vacuoles that protect them from phagocytosis.14,16,19
Colonization and infection of lymphoid tissue
Bacteria infect lymphoid tissue via the lymphatic circulation, and are found in macrophages in the liver, bone marrow and spleen, due to their tropism for these organs. Germs multiply there. The bacteria then spread throughout the body via the bloodstream. This propagation is responsible for bacteremia (abnormal presence of bacteria in the blood), which causes symptoms and can subsequently lead to sepsis (general infection of the body due to the spread of bacteria through the blood). Bacteria are found in blood cultures even when digestive symptoms have disappeared.14,15,20
The bacteria also colonize the kidney, gall bladder and Peyer's patches, once again via the bile. Lysis of the bacteria occurs, resulting in the release of endotoxin. This endotoxin causes toxic shock. These endotoxins are responsible for all the known symptoms of salmonella, particularly S.typhi Figure 6.14,15
Pathogenic effects and dysregulation of the immune response
Salmonella use various virulence factors to infect the host. These include pilus adhesins (small, very fine flagella), which help the bacteria adhere to the host's intestinal mucosa. Salmonella invade epithelial cells with the help of a group of chromosomal genes grouped together in pathogenicity islands called Salmonella Pathogenicity Island (SPI1, 2, or 3). These genes are activated when the bacteria come into contact with an epithelial cell of the intestinal mucosa. They are at the origin of proteins that modify the structure of the epithelial cells, allowing the bacteria to enter the cell. In this way, epithelial cells become phagocytic cells. The bacteria continue to multiply within the phagocytic cell, leading to lysis of the macrophage. In Salmonella typhi, the SPI genes encoding virulence factors, modifying host physiology or protecting the bacteria from attack by the host immune system, are different from those of other Salmonella.21–23 Salmonella multiplication within the macrophage is due to two groups of genes, one located on the SPI island and the other on a plasmid called the SSP virulence plasmid (serotype-specific plasmid). This plasmid is found in all Salmonella with a pathogenic serovar except Salmonella typhi.14,23,24
Salmonella are found intracellularly. They are less sensitive to host antibodies. In the case of immunosuppression, and in particular a drop in CD4+ T lymphocytes in an individual, Salmonella infection is more frequent and more persistent. Indeed, it is these CD4+ T lymphocytes that ensure the body's immunity to Salmonella.22 Lipopolysaccharides (LPS), present on the outer layer of the bacterial wall, play a major role in bacterial infection, activating the immune system and combating the bacteria. In addition, through the massive production of cytokines, they can become toxic to the infected organism.14,25
Humoral response
Salmonella's adaptive immunity requires both T-cell and immunoglobulin responses. The bacterium encounters phagocytes either in the gastrointestinal tract, or during penetration of the mucosal epithelium.26,27 Salmonella is then systematically disseminated in phagocytes to colonize organs such as the liver and spleen. Specific immunoglobulins produced by B cells can opsonize extracellular bacteria, which are phagocytized by macrophages.
Macrophages can act as antigen-presenting cells (APCs), presenting the antigen to the MHC (Major histocompatibility complex). After binding to the MHC, these peptides are presented to T cell receptors (TCRs). Other cells can act as antigen-presenting cells, such as dendritic cells and B lymphocytes. Dendritic cells recognize Salmonella LPS or flagellin through increased expression of MHC type II molecules and the co-stimulatory molecules CD80, CD86 and CD40 (CD = Differentiation Cluster). This process enables antigen presentation to TCD4 cells, providing a vital link between innate and adaptive immune responses.28 Antigen recognition by the TCR enables the activation and expression of CD4+ cells. CD4+ cells are absolutely essential for controlling Salmonella infection.
Once CD4+ cells are activated, they stimulate B lymphocytes to produce antibodies, as well as CD8 cytotoxic lymphocytes. The massive expansion of Salmonella-specific TCD4+ cells and the rapid acquisition of Th1 (help T lymphocytes 1) functions stimulates the release of immunomodulatory cytokines, such as INF-γ, TNF-α, IL-12, IL-18.29,30
Virulence factors
Salmonella enterica expresses various virulence factors common to most serovars. The main virulence factors are the type III secretion system (T3SS), lipopolysaccharide (LPS), surface polysaccharides, fimbrae, flagella, and pathogenicity islands. On the other hand, S. typhi, paratyphi and dublin are the only serovars to express capsular antigen (Vi). The virulence factors of Salmonella serovars are mainly encoded by Salmonella SPI pathogenicity islands Figure 7.31
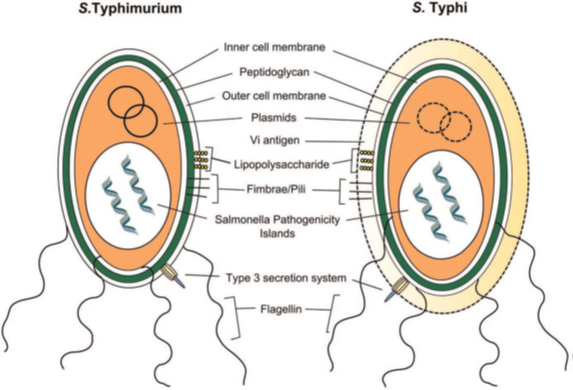
Figure 7 Virulence factors of S. Typhimurium and S. Typhi.31
Diseases associated with typhoid fever
Several diseases are associated with typhoid fever, such as:
Biology of the disease
Biological markers of the disease
The blood count of a person with typhoid fever shows normocytic, microcytic or macrocytic anemia; it may be normochromic or hypochromic; it is frequent, early, peaking in the third week. Leukoneutropenia (hyperleukocytosis indicates a complication). Hyperlymphocytosis is more marked from the 3rd septennium onwards. Low sedimentation rate. Liver function tests show moderate cytolysis
Role of the various phases of medical biology analysis
Pre-analytical phases
The pre-analytical phase is the first stage in the laboratory's core business macro-process, "performing a biological examination", and covers all stages from prescribing the analysis to presenting the sample on the analyzer. While this phase accounts for 57% of the time used, it is also the source of 85% of errors affecting test results.33
In the specific case of typhoid fever, after the consultation, the patient is taken to the sampling room for sampling, taking into account the analysis requested by the prescriber. Samples are then labelled and transported to the laboratory or centrifugation room, as appropriate. We will take into account the two characteristic tests for typhoid fever:
Analytical phase
Technical process used to obtain a biological analysis result.15
Coproculture examination comprises the following stages:34,35
Macroscopy: the appearance of the stool is noted.
Microscopy:
Fresh state: spread a small quantity of stool in physiological water between slide and coverslip to look for parasites, yeasts, crystals, leukocytes, red blood cells, etc.
Gram staining: to determine whether or not the bacterial flora has been disturbed. It involves spreading a stool suspension on a slide, drying and staining. Hard stools are diluted before spreading.
Blood culture examination involves the following steps.37–39
Incubate flasks at 37° for 7 days: visual readings are taken twice a day for the first 48 hours, and then only once a day for the next 5 days, to check for the presence of environmental disturbances caused by bacterial growth, hemolysis, colony coagulum at the bottom of the flask, gas production or the presence of colonies on agar plates. In the event of any suspicion of positivity, microscopic examination and culture are performed on the flasks.
Microscopic examination:
Post-analytical phase
This covers all stages after analysis, from transcription of the result, validation and transmission of the report, to interpretation.40
Biological diagnosis and different types of biological tests
Molecular biology tests
RT-PCR
PCR is the most interesting test in terms of specificity and sensitivity. The method is widely used for early diagnosis, as it can diagnose only a few Salmonella in the sample, and can give rapid results, not only reducing morbidity, mortality and the acquisition of chronic carrier status, but above all reducing disease transmission. This method makes it possible to determine Salmonella serovars using previously identified genomes. Results can be obtained in less than 48 hours.3,5,41
Microbial DNA sequencing
Genome sequencing is an important step towards a better understanding of pathogenic behavior. The first annotated genomic sequences of Salmonella were published in 2001. Serovars typhimurium (strain LT2) and typhi (strain CT18), widely studied for their medical importance, have genomes of 4,857,432 and 4,809,037 base pairs (bp), respectively.26,42,43 Technologies 454 (Roche) and Solexa (Illumina) enabled the sequencing of 19 isolates of serovar typhi in 2008.44 The complete sequences of 23 genomes are now known, including those of strains belonging to 15 distinct serovars of the enterica subspecies, one strain of the arizonae subspecies and one of the S. bongori species.45,46
In addition to annotated coding sequences (Open Reading Frame or ORF), other genome features are continually coming to light, including small regulatory RNA (sRNA) and very small membrane proteins, enhancing genomic analyses.47,78 Genome sequencing has enabled the creation of DNA microarrays consisting of ORFs49,50 and more complete tiling array microarrays.51 Despite the advances brought about by genome sequencing and annotation, gene function often needs to be verified and determined experimentally. The S. typhimurium genome contains some 4,500 genes, 1,000 of which remain unassigned. Comparative Genomic Hybridization (CGH) experiments, for example, have enabled the genetic content of non-sequenced clinical strains, often responsible for food poisoning, to be analyzed.45,52,53 This type of analysis has shown that the genome of strains of the same serovar may differ more from one another than strains of different serovars.
Immunoserology tests
The Widal and Felix serodiagnosis is performed by detecting anti-H and anti-O antibodies in the patient's serum, using the agglutination procedure. This test is no longer recommended for the diagnosis of typhoid fever, due to its poor diagnostic performance. Because of its low sensitivity, the Widal and Felix serodiagnosis may be negative even in cases confirmed by blood culture. Indeed, in cases of typhoid fever, anti-O antibodies appear from day 8, peaking on day 14. The specificity of the Widal and Felix test is no better. As this is an indirect test that detects serum antibodies, antibody persistence can be observed in a healed, healthy subject. In addition, cross-reactivity with other fever etiologies is common.54
Biochemical tests
Biochemical parameters are rarely requested, but in the event of complications, liver function tests are required to assess normal liver function. They are even less significant, and are rarely sought in routine practice: moderate elevation of blood transaminases (especially early in the course of the disease), lowering of total cholesterol, inverted albumin/serum globulin ratio, increased serum lactic dehydrogenases, which would be of diagnostic interest at an early stage, and whose regression under treatment would indicate its efficacy.55
Hematology tests
A complete blood count (CBC) is taken from the patient's blood to assess elevation or reduction of white blood cells, hemoglobin and other hematological parameters (hematocrit, mean corpuscular volume, platelets, etc.).
Sedimentation rate is normal or only slightly increased. If it is moderately accelerated, this is a significant sign of interest in a patient presenting with a high, long-lasting fever.
Bacteriological tests
Two bacteriological tests are used to diagnose typhoid fever:
Blood culture: This is the most important test for diagnosing typhoid fever. As there is little Salmonella in the bloodstream, blood cultures must be repeated. In the absence of antibiotic treatment, they are positive :
Isolated bacteria are identified as salmonella by their biochemical characteristics. The species involved is then determined by its antigenic characteristics. An antibiogram completes the examination.
Coproculture is performed on selective medium, before and after preculture on enrichment medium. Given the low number of Salmonella excreted in stools, this test must be repeated, but is often negative. Coproculture is therefore not the best way of making a biological diagnosis of typhoid fever. On the other hand, at the end of treatment, it is a good way of ensuring that the patient has not become a chronic carrier of Salmonella, and is therefore not a source of contamination for those around him.4,12
Responses of diagnostic and research laboratories and public health players to the disease at national, regional, continental and global level
To combat typhoid fever effectively, national disease surveillance centers have been set up in various countries.54 A number of actions have been or need to be taken in each country:
Technological innovations and disease
Vaccines have been developed to combat the disease effectively.9,54,56 Two types of vaccine are available:
New prospects are emerging for vaccines that could prevent epidemics in regions where the disease is becoming difficult to treat. Work is currently underway on a polysaccharide Vi-rEPA vaccine.
The aim of this review is to update data on typhoid fever and the contribution of medical biology to the diagnosis of the disease. Searches were carried out in the Pub Med and Google Scholar databases for articles and some of their references. 57 articles were consulted, and it emerged that the disease is currently distributed in developing countries. Typhoid fever is an invasive disease with several phases before lymphoid tissue is affected, so early management is essential. Mortality varies with age, and can be as high as 20%. Clinical management necessarily involves biological diagnosis, from pre-analytical to post-analytical phases. Compliance with the various phases is vital to avoid errors in the results. The characteristic tests for the disease are coproculture and blood culture. They must be prescribed within the first few hours of illness to ensure effective management. Authorities, especially in developed countries, have put in place measures to eradicate the disease, such as compulsory declaration, hygiene and vaccination. Vaccines have been developed to minimize infection by salmonella bacteria.
Typhoid fever is a potentially fatal infection caused by Salmonella typhi bacteria. It is generally spread through contaminated food or water. Once the bacterium has been ingested, it multiplies and enters the bloodstream after several phases, leading to complications that can be digestive, cardiovascular, neurological, etc., without early treatment. The aim of this paper is to review the literature on the germ responsible for typhoid fever, its pathophysiology and the various laboratory techniques for isolating Salmonella bacteria. Mortality is estimated at 1-4% in children. In the absence of any treatment, it is around 1-20%. Biological diagnosis is based essentially on the isolation of S. typhi from stools after coprocultures or from blood cultures. Molecular biology tests have shed light on the bacterial genome. In order to combat typhoid fever effectively, national epidemiological surveillance centers have been set up by the most developed countries to deal with any epidemics caused by S. typhi. These centers work closely with laboratories to ensure rapid identification of the germ.
We thank all those who have closely contributed or by far to the development of this review.
The authors state do not have any conflict of interest.

©2024 Hoinsou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.