
Research Article Volume 7 Issue 1
Oscillations through a barrier tunneling potential for a particle in a central potential (double potential well)
Hasan Hüseyin Erbil,
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Ahmet Saim Selvi
Department of Physics, Faculty of Sciences, Ege University, 35100 Bornova – İzmir, Turkey
Correspondence: Hasan Hüseyin Erbil, Department of Physics, Faculty of Sciences, Ege University, 35100 Bornova – İzmir, Turkey, Tel +90 533 481 9918,
Received: January 08, 2024 | Published: January 23, 2024
Citation: Erbil HH, Selvi AS. Oscillations through a barrier tunneling potential for a particle in a central potential (double potential well). Open Access J Sci. 2024;7(1):5-13. DOI: 10.15406/oajs.2024.07.00208
Download PDF
Abstract
In some molecules such as ammonia, double vibrations are observed at small energies. These small vibrations are thought to arise from double well potentials. To explain these small energy gaps, double potential wells are investigated, and complex calculations are made. Until recently, since the exact solution of the Schrödinger equation was not known, approximate solutions were always obtained. The exact solution of the Schrödinger equation is now known. In previous studies, a simple procedure for the general solution of the radial Schrödinger Equation has been found for spherical symmetric potentials without making any approximation. In this article, the Schrodinger equation was solved with this new solution method. It has been applied to the oscillations through a barrier tunneling potential for a particle in a central quadratic deformed potential well (double potential well). It is seen that there is no need to look for complex potentials to explain the double wells observed in small vibrations in ammonia and similar molecules, they were compared with experimentally measured energies. The results were found to be fully compatible. It has been seen that there can always be double well potentials at small energies for all molecules, atomic nuclei, and similar particles with angular momentum other than zero.
Keywords: central potentials, radial equation, spherical symmetric potential, oscillations through a barrier tunneling, double potential well, small vibrations
Introduction
Although the radial Schrödinger Equation (SE) for some simple spherical symmetric potential is solved, an exact solution is not possible in complicated situations, and it must be then resorted to approximation methods. For the calculation of stationary states and energy eigenvalues, these methods include perturbation theory, the variational method and the WKB approximation. Perturbation theory is applicable if the Hamiltonian differs from an exactly solvable part by a small amount. The variational method gives a good estimate of the ground state energy if one has a qualitative idea of the form of the wave function and the WKB method is applicable in the nearly classical limit. In one of the previous studies, it has been achieved a simple method for the exact general solution of the radial SE for spherically symmetric potential well of any form without making any approximation. This simple solution method has been applied to many spherical symmetric potential.1,2 In this study, we have applied this simple method to solve the oscillations through a barrier of potential (double potential well). It was applied to the ammonia molecule, and it was seen that the values calculated with experimental measurements agreed very well. It is seen that there is no need to look for complex potentials to explain the double wells observed in small vibrations in ammonia and similar molecules, and that there will be double wells in small vibrations in all molecules, atomic nuclei, and similar particles with non-zero angular momentum.
Radial Schrodinger equations for spherical symmetric potentials and their solution (general solution)
The SE in three dimensions is given as follows:
Δψ(→r)+2mℏ2 [E−V(→r)]ψ(→r)=0
(1)
Where, E and V are the total and potential energies, respectively, m is the mass or reduced mass of particle. The spherical polar coordinates (r,θ,ϕ)
are given as follows:
X=r sin(θ) cos(ϕ), y=r sin(θ) sin(ϕ), z=r cos(θ)
These coordinates appropriate for the symmetry of the problem. The Equation (1), expressed in these coordinates, is as follows:
[∂2∂r2+2r∂∂r]ψ(r,θ,ϕ)+1r2^L2(θ,ϕ)ψ(r,θ,ϕ)+2mℏ2[E−V(r,θ,ϕ)]ψ(r,θ,ϕ)=0
(2)
ˆL2(θ,ϕ)=∂2∂θ2+cotg(θ)∂∂θ+1sin2(θ)∂2∂ϕ2
The potential energy of a particle which moves in a central and spherically symmetric field of force depends only upon the distance r between the particle and the center of force. Thus, the potential energy should be such as V(r,θ,ϕ)=V(r)
. Solution of the Equation (2) can be found by the method of separation of variables. To apply this method, it is assumed that the solution is in the form of
Ψ(r,θ,ϕ)=R(r)Y(θ,ϕ)=R(r)|jm >
(3)
in which R(r) is independent of the angles, and Y(θ,ϕ)
and |jm >
are independent of r. By substituting Equation (3) into Equation (2) and rearranging, the following Equation is obtained. In the Equation (3),m is not mass, it is magnetic quantum number.
[∂2∂r2+2r∂∂r]R(r)+{2mℏ2[E−V(r)]−Cr2}R(r)=0
(4)
ˆL2(θ,ϕ) Y(θ,ϕ)+C Y(θ,ϕ)=0
(5)
Here, C is a constant. Equation (5) is independent of the total energy E and of the potential energy V(r) therefore, the angular dependence of the wave functions is determined by the property of spherical symmetry, and admissible solutions of Equation (5) are valid for every spherically symmetric system regardless of the special form of the potential function. The solutions of the Equation (5) can be found in any quantum mechanics and mathematical physics text-books and the solutions are known as spherical harmonic functions, Y(θ,ϕ)=Ylμ(θ,ϕ),
where C=l(l+1),l=0,1,2,3,...
are positive integer numbers and μ=−l, −l+1,...0...+l.
Equation (4) is the radial SE (Schrödinger Equation). Substituting C=l(l+1)
and R(r)=F(r)/r
into Equation (4), the radial wave Equation is obtained as follow:
{d2dr2+2mℏ2[E−U(r)]}F(r)=0
;d2F(r)dr2+[α−Ue(r)=0
(6)
Here α=2mℏ2E
; U(r)=V(r)+ℏ22ml(l+1)r2
is the effective potential energy, and Ue(r)=2mℏ2U(r)
. here m is mass or reduced mass of particle.
Equation (6) is one dimensional differential equation. The solution of this one-dimensional differential equation has been given in.1,2 The F(r) functions have been found by using the same procedure that is explained in these references. Two of these solutions are given as follows:
F(r)=cosh[k r] [A ei G(r)+B e-iG(r)] ;
F(r)=sinh[k r] [A ei G(r)+B e-iG(r)]
(7)
Here,
For α>Ue(r),
(bound state), k=√−α , G(r)=∫√−Ue(r) dr ; [G(r)
real function]. For α>Ue(r),
(unbound state), k=√α, G(r)=∫√−Ue(r) dr .
r1 and r2
are the roots of the following Equation:
α−Ue(r)=0 or E−U(r)=0
(8)
In some cases, using the following quantities can provide more conveniences:
d=r2− r1, (r1< r2); r0=(r1+ r2)/2; r1=r0 −d/2 ; r2=r0+d/2.
If one takes (r−r0)
instead of in the above functions, these can also be used when obtaining the solutions of the Equation (6). The A and B coefficients of the functions are determined by using the boundary and normalization conditions.
In bound states (in the wells), the normalized wave functions are as follows [ is taken as real function]:
Fs(r)=A cos[k r] e±i G(r)
Fa(r)=B sin[k r] e±i G(r)
(9)
or
Fs(r)=A cos[k (r−r0)] e±i G(r−r0)
Fa(r)=B sin[k (r−r0)] e±i G(r−r0)
(10)
A = B = √2/d=√2K/q;
; (s: symmetric; a: antisymmetric).
The bound state energies are given by the solution of the following Equation:
K [r2(E)−r1(E)]=Kd=q
;K=√|α|=√2 mℏ2|E| .
(11)
Equation (11) is the quantization condition of energy in bound states. For q=2 the ground state (minimum energy) occurs; for q=n π, (n=1,2,3,...),
the excited states occur. We have symmetric states for odd integer values of n and antisymmetric states for even integer values of n.
Solution of Schrodinger equation in double potential wells
Consider a particle with mass or reduced mass m is in the central potential of V(r) in a coordinate system at the point (0,0) of the coordinate start. The variable r of the potential in this coordinate system is always positive. If the spin of the particle is also considered, the total angular momentum quantum number j should be taken instead of L. Thus, the effective potential is U(r)=V(r)+b/r2 ; [b=ℏ2 j (j+1)/(2m)]
. This effective potential is usually a parabolic function in the bound states. In the bound states, the total energy is always negative and consequently the effective potential is also negative. Therefore,U(r)<0
should be in the bound states. When mass m vibrates in this effective potential, the term b of the potential creates an obstacle that tries to prevent vibration motion. This potential comes from the rotation of the mass m, that is, from the centrifugal motion. If there is no rotation, this term is zero. The solution of the Equation U(r)=−E or |U(r)|=E
are r1, r2,r3 and r4
values, depending on E. If the values (r3 and r4)
are real and non-zero, there is a potential barrier in the potential well. If this Equation is solved at a point r=r0
, these roots are written also as follows: r1=r0−d1/2
and r2=r0+d1/2
and r3=r0−d3/2
and r4=r0−d3/2
. Here d1=(r2−r1),d3=(r4−r3)
and r0=(r1+r2)/2=(r3+r4)/2
. If the origin of the coordinate system is taken as the point r0
, these are r1=−d1/2 and r2=−d1/2
and r3=−d3/2
and r4=−d3/2
. The points r1 and r2, and r3 and r4
are the classic turning points of the U(r)
potential. Since U(r1)=U(r2) and U(r3)=U(r4)
are equal at the potential of the classical turning points. Thus, the following Equations can be written:
U(−d1/2)+U(+d1/2)=2E
U(−d3/2)+U(+d3/2)=2 E
Since E= mh q2/d2i(E) , ( mh=ℏ22 m ) .
these Equations can also be written as follows:
U(−d1/2)+U(+d1+2)=2 mh q2/d2i(E)
U(−d3/2)+U(+d3+2)=2 mh q2/d23(E)
The solution of these two Equations has the same energy. It is therefore sufficient to solve one of these two Equations to find the energy values. When these Equations cannot be solved analytically, numerical solutions are made, and energy values are found. When one of these two Equations is solved, two energies based on q are obtained. One of these energies (+) and the other (-) becomes and their absolute values are equal. The (-) sign indicates that the particle is bound. For q=2 the ground state (minimum energy) occurs; for q=n π, (n=1,2,3,...),
the excited states occur. We have symmetric states for odd integer values of and antisymmetric states for even integer values of . This problem is useful in some cases if it is solved in two wells.
Conversion of potential into two parts (double well)
Solutions become easier when you divide a given potential into two parts. This separation process will be given here. If the potential is given as V(r)=V0(r)−V00,[V0(r)>0 and V00> 0], the effective potential is U(r)=V0(r)−V00+br2<0
in the bound states. Here, −V00
is the depth of the potential well. Let us find the maximum and minimum values of this effective potential U(r)
et the roots of the Equation U'(r)=0,
rm1 and rm2 be. r0=(rm1+rm1)/2
is the point where the potential receives the smallest values U(rm1) and U(rm2)
and the largest value U(r0)
. Let U0=U(r0) −V00
Thus U(r)=V0(r)−U+br2<0
can be written. By solving this U(r)
potential directly, energy values and wave functions can be found. However, if this potential is divided into two parts with an obstacle in the middle, there may be some convenience. The obstacle in the two potential wells comes from rotational energy and the potential [V0(r)−U0]
. Therefore, the U(r)
potential can be written as the sum of two parts as follows:
U(r)=V0(r)−U0+br2=Uw(r)+Ub(r)
;[Uw(r)=V0(r)−U0 ; Ub(r)=br2]
(12)
Here, the Uw(r)
potential is the vibration part of the U(r)
potential, and the Ub(r)
potential is total of the rotational and the other obstacle potential parts of the potential U(r)
.U0
is the depth of the potential well. If the coordinate start is taken at the point (r0,−U0)
in this new coordinate system, Ub(r)=br2
and Uw(r)=V0(r)
. Thus, the effective potential is written as follows:
U(r)=V0(r)+br2=Uw(r)+Ub(r)
;[Uw(r)=V0(r) ; Ub(r)=br2]
(13)
The graph of this potential is shown in Figure 1. (Shape of the U(r) function
(r0,−U0)
in the coordinate system). In this way, three domains I, II, III are obtained. Thus, by solving the Equation (13), E energy values are found.
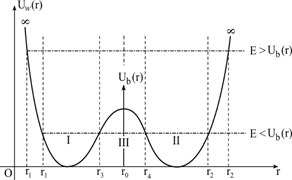
Figure 1 The plot of the potential U(r) in the form of two wells; the points r1,r2,r3,r4 are classic turning points.
Solution of the equation of [U(r)=Eq]
The functions Uw(r) and Ub(r)
are symmetrical functions according to point r0
. The Ub(r)
function is an obstacle function located in the midpoint of the well Uw(r)
function. The quantities (r1,r2,r3,r4)
that are the solution of the Equation U(r)=E
are depending on r0
. As seen in Figure 1, the roots of the Equation, U(r)=E
are (r1, r2, r3, r4)
and the roots of Ub(r)=E
are (r3 , r4),
the roots of Uw(r)=E
are (r1 , r2)
. From there, the following values are obtained:
r1+r22=r3+r42=0 ; d1=r3−r1 ; d2=r2−r4 ; d1=d2 ; d3=r4−r3 ; d=r2−r1
[d1, d2, d3]
are respectively the widths of the region (I,II,III)
. Since d1=d2
, it is sufficient to make a solution in one of the regions (I) and (II). Particle is unbound in the region , it cannot remain in this area continuously, it can pass from region II to region I or from region I to region II by tunneling with equal probability. Here the probability of passing coefficient is calculated.
Example: Three axial deformed harmonic oscillator potential (anisotropic harmonic oscillator potential) (Nilson model in the nuclear physics)
Effective potential
The nucleus is assumed to have a spherical shape in the shell model. Therefore, particles move in a spherically symmetric potential. There are, however, convincing arguments that nuclei with the neutron and proton numbers sufficiently far from the magic numbers have no spherical symmetry ellipsoidal shapes. In this case, it is said that the deformed shell model. In deformed shell model calculations, it is used the three-dimensional anisotropic harmonic oscillator potential which is given as follows:
V0(x,y,z)=12μ(ω2xx2+ω2yy2+ω2ZZ2)
(14)
Here,μ is mass or reduced mass. In the case of deformed nuclei, it is generally restricted to axially symmetric nuclei and it taken the z -axis as symmetry axis. So, it is accepted ωX=ωy=ω⊥≠ωz
in the anisotropic harmonic oscillator potential. The motion of a particle in an axially symmetric potential, with additional symmetry plane, perpendicular to symmetry axis was described by Nilsson. The no-spherical nuclei have the shape of an ellipsoid of revolution. It is, however, possible that some transitional nuclei have shapes of a three-axial ellipsoid. It is also possible that the shapes of excited states differ from the ground state shapes and that some exited states have three axial ellipsoidal forms. In the three axial cases, ωX≠ωy≠ωz
. The no-axial shape is characterized by two parameters ε and γ
. For ε >0, γ=00
situation corresponds to the axially symmetric prolate ellipsoid,γ=600
corresponds to the oblate ellipsoid. When γ≠00 and γ≠600
, the ellipsoid has no axial symmetry, and the projection quantum number of the total angular momentum on any axis is not one conserved quantity. The angular frequencies ωx,ωy,ωz
relate to the deformation parameters ε and γ
by the following expressions:
ωx=ω0(ε,γ)[1−23ε cos(γ+2π3)]
;ωy=ω0(ε,γ)[1−23ε cos(γ+2π3)]
ωz=ω0(ε,γ)[1−23ε cos(γ)]
(15)
If it is required a constant volume as deformation changes, it needs:
ωxωyωz=ω300
(16)
From Equation (16), we get the following value:
ω0(ε,γ)=3ω00[27=9ε2−2ε3 cos(3γ)]−1/3
(17)
As can be seen from Equation (17), if ε=0,
isotropic state is obtained. Let us express the potential given in Equation (14) the following spherical coordinates:
x= r sin(θ) cos(ϕ), y=r sin(θ)sin(ϕ), z=r cos(θ)
(18)
If the potential given in Equation (14) is calculated by considering Equations (15), (16) and (17), the following function is obtained:
V0(r,ε,γ)=12μω200r2[A(ε,γ) sin2(θ) cos2(ϕ)+B(ε,γ) sin2(ϕ) sin2(ϕ)+C(ε,γ) cos2(θ)]
(19)
A(ε,γ)=[3+2ε sin(π6+γ)]2[27=9ε2−2ε3 cos(3γ)]2/3;
B(ε,γ)=[3+2ε sin(π6−γ)]2[27−9ε2−2ε3 cos(3γ)]2/3;
C(ε,γ)=[3−2ε cos(γ)]2[27−9ε2−2ε3 cos(3γ)]2/3;
(20)
β2=A(ε,γ) sin2(θ) cos2(ϕ)+B(ε,γ) sin2(θ) sin2(ϕ)+C(ε,γ) cos2(θ)
β2=A(ε,γ) [1− cos2(θ)] cos2(ϕ)+B(ε,γ) [1− cos2(θ)][1− cos2(θ)]+C(ε,γ) cos2(θ)
β2=B(ε,γ) [C(ε,γ)−B (ε,γ)−{A(ε,γ)−B(ε,γ)} cos2(ϕ)] cos2(θ)+[A(ε,γ)−B(ε,γ)] cos2(θ)
(21)
If γ is exceedingly small, then sin(γ)≈0
and A≈B
(symmetric ellipsoid form) can be taken. Thus, β2
can be taken as follows:
β2=B(ε,γ)+ [C(ε,γ)−B (ε,γ)] cos2(θ)=B(ε,γ)+[C(ε,γ)−B(ε,γ)][13+23√4π5Y20]
(22)
Y20
is spherical harmonic function. Thus, the anisotropic harmonic oscillator potential becomes as follows:
V0(r,ε,γ)=12μω200r2β2(ε,γ)
(23)
With this potential, the effective potential U(r,ε,γ)
is obtained as follows:
U(r,ε,γ)=V0(r,ε,γ)+ℏ22μr2j(j+1)=12μω200r2β2(ε,γ)+ℏ22μr2j(j+1)
(24)
Here, ω00
is the isotropic oscillator angular frequency and total angular momentum quantum number. The Coulomb potential must be added to the potential given in Equation (24) when the charged particle levels are calculated. The Coulomb potential in the spherical case, neglecting the effect of the surface, is as follows:
Vc(r)=(Z−1) e2r{3 r2 R0−12(rR0)2 , for r≤R01 , for r>R0 }
Here,R0
is the radius of the spherical nucleus and Z is the charge number. Thus, in the nucleus, r≤R0
, we have as follows:
Vc(r)=bc+ac r2
; [ac=− (Z−1)e22 R30 , bc=3 (Z−1)e22 R0 ]
(25)
In the quadratic deformed case, the Coulomb potential is obtained as follows:
Vc(r)=3(Z−1)e22R0{−8.9723−3.3241 ε+8.14235√1.5+ε+acr2, for r≤R0}
Vc(r)=(Z−1)e2R0{R0r−4.76142−1.58717 ε+3.88768√1.5+ε, for r>R0}
Because in the nucleus r≤R0,
in the case quadratic, the Coulomb potential can be rewritten as follows:
Vc(r)=bc(ε)+ac r2
;
[ac=−(Z−1)e22 R30, bc (ε)=3(Z−1)e22 R0(−8.9723−3.3241 ε+8.14235√1.5+ε)
(26)
It is seen that for ε=0
, Equation (26) is equal to Equation (25). On the other hand, the total wave function is ψ(r,θϕ)=R(r)|ljm>=F(r)r|ljm>
and the radial SE is written as follows:
d2F(r)dr2|ljm>+2μℏ2[E−U(r,ε,γ)]F(r)|ljm>=0
;d2F(r)dr2|ljm>+2μℏ2F(r)[E−U(r,ε,γ)]|ljm>=0
(27)
In Equation (27), [E−U(r,ε,γ)]|ljm>
is calculated as follows:
[E−U(r,ε,λ)]|ljm> =E|ljm>−U(r,ε,γ)|ljm>
U(r,ε,γ)|ljm> =[12 μ ω200r2 β2(ε,γ)+ℏ22 μ r2j(j+1)]|ljm>
β2(ε,γ)]|ljm> =β2|ljm>={B(ε,γ)+[C(ε,γ)−B(ε,γ)][13+23√4π5Y20]}|ljm>
(28)
β2(ε,γ)]|ljm> = β2|ljm>={B(ε,γ)+[C(ε,γ)−B(ε,γ)]cos2(θ)} |ljm>
If we calculate Y20|ljm〉 =a20|ljm〉
in Equation (28), by the Wigner-Eckart theorem, we have found the following value:
a20(l,j,m)−√π2l+1j+j2−3m22j(j+1)=a20
Thus, β2(ε,γ,a20)
are written as follows:
β2(ε,γ,a20)=B(ε,γ)+[C(ε,γ)−B(ε,γ)][13+23√4π5a20]
(29)
So, we have found the effective potential for anisotropic harmonic oscillator as follows.
U(r;ε,γ,a20)=12μω200β2(ε,γ,a00)r2+ℏ22μr2j(j+1)=ar2+br2=U(r)
(30)
a=10μω200β2(ε,γ,a20)=12μω2;b=ℏ22μj(j+1);ω2=ω200β2(ε,γ,a20)
(31)
In the case of electric charged particle, the Coulomb potential should be also added to this effective potential.
Solution of the equation of [U(r)=Eq]
Consider Uw(r)=a r2
and Ub(r)=ℏ22 μ j (j+1)r2=br2 , [b=mh j (j+1) , mh=ℏ22 μ ].
Thus, according to (30), we have:
U(r)=V0(r)+ br2=Uw(r)+Ub(r) ;
[ Uw(r)= V0(r)=a r2 ; Ub(r)= br2]
.
Solution of the Equation U(r)=Eq
gives the following values:
r1=−√Eq+√E2q−4 a b2 a ;
r2=√Eq+√E2q−4 a b2 a ;
r3=−√Eq−√E2q−4 a b2 a ;
r4=√Eq−√E2q−4 a b2 a
.
r1+r22=r3+r42=r0=0 ; d1=r3−r1 ; d2=r2−r4 ; d1=d2 ; d3=r4−r3 ; d=r2−r1
.
Now, we will solve this problem in three cases as follows.
Solution of the case Eq>Ub(r)
In this case, the following equation can be written according to the quantization condition of energy:
K d=Eq or K2d2=E2q ; [K=√2 μ Eqℏ2]
The following energy value is obtained from the solution of this equation.
Eq=√a ℏ2q22 √2 μ √ℏ2q2−8 μ b ; [Eq>Ub(r)]
(32)
If the energy Eq
does not depend on the angular momentum quantum number j, i.e., rotation, energy is purely vibrational energy. In this case, b=0 and Eq=12ℏ ω
.
Solution of the case Eq<Ub(r)
In this case, since d1=d2
, equal energy values are obtained in (I) and (II) regions. So, the following equation can be written according to the quantization condition of energy:
K d1=Eq or K2d21=E2q ; [K=√2 μ Eqℏ2]
The following energy values are obtained from the solution of this equation.
E(1)q=√a μ (4 b μ+ℏ2q2)+δ(a,b,q)μ
;E(2)q=√a μ (4 b μ+ℏ2q2)−δ(a,b,q)μ
(33)
δ=2√2 a2b μ3(2 b μ+ℏ2q2)
If the values of b are put in place, the following values depending on (a, j, q) are obtained:
Eq1(a,j,q)=√a ℏ2(2j+2j2+q2)μ+δμ
; Eq2(a,j,q)=√a ℏ2(2j+2j2+q2)μ−δμ
. (34)
δ(a,j,q)=2aℏ2μ√j(1+j)(j+j2+q2)
Then, in case Eq<Ub(r)
, there are two equal energy values in regions (I) and (II). As seen in Figure 1, the Ub(r)
barrier potential located between (I) and (II) potential wells divides the energy of the particle into two. While the particle is oscillating in these wells, when the particle reaches the r3 (or r4) point, some of its energy passes from the III region to the II (or I) region by tunneling, and some of it is reflected from the r3 (or r4) point. This transition is equally likely for both parties. (This passing probability will be calculated below). Thus, the potential barrier divides the energy of the particle in two. The rotational potential due to angular momentum always splits the energy into two parts. This phenomenon may be a way to find the angular momentum of a particle. When j=0,(δ=0)
in (34), there is only one energy. So, the Ub(r)
barrier cuts a single energy into two parts. These double energy splits are observed at small vibrations in some molecules such as ammonia. Many double wells are proposed to explain these energy splits, although there is no need for them. Because this energy splitting occurs in small vibrations for all particles with angular momentum.
Solution of the case Eq=U(r)
, [In one potential well]
We consider the effective potential given here as a whole. We will solve the two wells at the bottom of the potential well in general, without considering them separately.
U(r)= V0(r)+ br2=Uw(r)+Ub(r) ;
[ Uw(r)= V0(r) ; Ub(r)= br2 ; V0(r)=a r2]
Solution of the Equation U(r)=Eq
gives the following r values:
r1=−√Eq+√E2q−4 a b2 a ; r2=√Eq+√E2q−4 a b2 a ;
r3=−√Eq−√E2q−4 a b2 a ; r4=√Eq−√E2q−4 a b2 a
;
d1=r2−r1 ; d3=r4−r3
.
Positive root of Equation d21 [U(r1)+U(r2)]=2 mh q2
is Eq1=√a mh q22 √mh q2−4 b=q2 ℏ ω 4 √q2−4 j (j+1)
.
Positive root of Equation d23 [U(r3)+U(r4)]=2 mh q2
is
.
From here it is seen that
. Since the particle is not bound in zone so energy is obtained as follows.
(35)
It is seen that the energy of (32) and the energy of (35) are the same for
. We have for
the minimum (ground) state energy; for
the exited state energies. We have symmetric states for odd integer values of n; antisymmetric states for even integer values of .
Here the solutions are made for the potential
and all solutions could be done analytically. If the potential is not a potential that can be solved analytically (as Saxon-Wood potential, trigonometric potentials etc.), numerical solutions can be made in the same way.
Finding wave functions
If the potential
potential is an isotropic harmonic oscillator potential, therefore, the mass or reduced mass m in zone (I) makes a harmonic motion. If there were no (III) barriers, there would be only one energy value. So, the energy value would be corrupt. With such a two-well solution, corruption is eliminated. Therefore, total energy would be 32 or (35) if there were not the
obstacle at the point
. According to the function (10), the radial normalized wave functions can be written as follows:
;
(36)
For the state of
, the independent of time and time dependent normalized wave functions are as follows, respectively:
(r)=A cos [K r]
;
Fa (r)= B sin [K r]
.
. (37)
The independent of time and time-dependent total normalized wave functions are as follows, respectively:
(38)
Let us consider a general solution of the type of time-depending functions:
(39)
(a: antisymmetric; s: symmetric). Here, both states, symmetric and antisymmetric, are equally probable. Let us calculate the probability density of presence as follows:
(40)
If the wave functions of the energy states
given in the formulas (34) are
, respectively, and the general wavefunction and the probability density of presence will be as follows:
When the cosine is equal to 1, we have
and this corresponds to a state where the probability of finding the particle in domain I is maximum. When the cosine is equal to -1, we have
and this corresponds to a state where the probability of finding the particle in domain I is minimum. The expression (39) and (40) must be interpreted by saying that it is a state where particle oscillates from the left bowl (domain I) to the right bowl (domain II). The frequency of this oscillation is
To perform such an oscillation corresponding to the energy variation (
), the particle must receive energy from the outside, for example by placing it in an electromagnetic field having the frequency f. Such an oscillation is not possible classically because the energy supplied
is insufficient for the particle to cross over the hump of the potential
. But, according to quantum mechanics, by tunneling through the III region, these oscillations are possible. It is observed similar states in ammonia
, and similar molecules which have the shape of a pyramid. This is called rotational symmetry. If
, there is one energy value and one wave function; so, there is no energy splitting. If
, there are always two energies and two wave functions; so, there is an energy splitting.
Calculation of the transmission coefficient through the zone (III)
The particle is unbound state in the region (III). From the solution of the
Equation,
values depending on
are obtained. The solution to this Equation (at the point
) gives the following values
. From here, the width of the obstacle
is found:
. The energy
is found by the Equation (33). In the region III,
thus the particle cannot remain stable in zone , it can pass from region II to region I or from region I to region II by tunneling with equal probability. Here the probability of passing coefficient is calculated. The tunneling probability coefficient (or transmission coefficient) is given by the following formula:3–5
Here, the width of the potential barrier d,
E energy,
,
barrier potential and
. Here, according to our quantities, these quantities are as follows:
;
;
;
.
If
is pair,
If
is odd,
Thus, if
, the coefficient transmission is obtained as follows:
;
In regions (I) and (II), energy satisfies the quantization condition
. In zone (III), the particle is not bound. But the energy will be equal at
points and
quantization condition gets also in the (III) region. Therefore, it is
. So, it is obtained as
(41)
Numerical calculations
The energy values of some triaxial harmonic oscillator states calculated according to the formula (34) are given in Table 1. In this table, the parameters are taken arbitrarily to see how the energy values vary according to these parameters.
|
States
q = 2
|
Eq1
ε=0
γ=00
|
Eq2
ε=0
γ=00
|
Eq1
ε=0.35
γ=00
|
Eq2
ε=0.35
γ=00
|
Eq1
ε=0.35
γ=300
|
Eq2
ε=0.35
γ=300
|
| f7/2 5/2 |
4.30371
|
4.98456
|
4.52261
|
5.23809
|
4.1478
|
4.80398
|
| f5/2 5/2 |
3.36143
|
5.04564
|
3.88143
|
5.82618
|
3.4523
|
5.18205
|
| g9/2 5/2 |
5.26168
|
5.64722
|
5.28032
|
5.66723
|
4.92295
|
5.28368
|
| g7/2 5/2 |
4.30371
|
4.98456
|
4.51152
|
5.22525
|
4.14115
|
4.79628
|
| d5/2 5/2 |
3.36143
|
5.04564
|
3.95404
|
5.93517
|
3.49732
|
5.24963
|
| h11/2 5/2 |
6.22978
|
6.48175
|
6.12706
|
6.37487
|
5.7554
|
5.98818
|
| h9/2 5/2 |
5.26168
|
5.64722
|
5.29317
|
5.68102
|
4.93054
|
5.29182
|
| i13/2 5/2 |
7.20478
|
7.38356
|
7.02208
|
7.19631
|
6.6188
|
6.78303
|
Table 1 Some energy values of the three-axial harmonic oscillator calculated according to (34),
Application to ammonia molecule
The ammonia molecule consists of one nitrogen and three hydrogen atoms. The effective potential curve becomes as shown in Figure 1. The hydrogen atoms define the orientation of ammonia. If the nitrogen atom is situated on the right side of potential barrier there is a chance that it can overcome the barrier and move to the other side. The movement of nitrogen atom consists of left and right vibrations and a slow drift between left and right zones of the potential. The classical turning points for the ground vibrational state,
or
, are indicated as well in Figure 1. Due to tunneling through the potential barrier each vibrational level is split in two symmetrical components. As seen from formula (34), masses are not needed to calculate energies. Masses are within the angular frequency
. Therefore, from the Equation (31)
, and must be explicitly calculated based on the mass (hence ω). But the mass is also calculated here to explain how the problem is. The masses and total angular momentums of hydrogen and nitrogen atoms are given as follows, respectively.
and
;
;
.
The reduced mass of three hydrogen atoms and one nitrogen atoms is as follows:
;
.
The relative total angular momentum of a hydrogen atom and other atoms are as follows:
Since
is not suitable for the smallest energy
it is sufficient to take
. Since
for the smallest energy state
will be taken here. With these data, the following energy values are found by the formulas (34).
Ground state energy
According to the formulas (34):
.
According to the formula (35):
(Since formula (34) gives the energy of the excited states, it is necessary to take
when using this formula when calculating the ground state energy).
Energies of excited states
According to the quantization condition of energy:
for the ground state,
for the excited states. The smallest energy (ground state energy) value measured in the ammonia molecule is
;
.6 Some excited energy values calculated by taking this energy value as zero are given in Table 2. In this table, the first and second columns experimental energies; third, fifth and seventh columns
parameters giving the energy split
; the fourth, sixth and eighth columns are the energies found by the parameters
, according to the formulas . The
values are obtained by solving the equations
. As can be seen in Table 2, it is understood that the energy is divided into two parts in the small energy states observed in the ammonia molecule. The measured energies and the calculated energies are the same. Very complex double wells are sought to explain these small vibrations. However, if there is angular momentum other than zero, it is possible to observe small vibrations in each potential well. When the angular momentum is different from zero, splitting occurs even in high energy states, but it may not be possible to measure it because the difference between the splitting energy values is very small.
|
Eexp
(unit cm-1)
|
Eexp
(unit eV)
|
γ=0
ε=0.00
q1,q2 |
Ecal
(unit eV)
|
γ=0
ε=0.10
q1,q2 |
Ecal
(unit eV)
|
γ=0
ε=0.20
q1,q2 |
Ecal
(unit eV)
|
|
0.0
|
0.0
|
|
0.0
|
|
0.0
|
|
0.0
|
|
0.79340
|
9.813x10-5
|
2.36603q1
1.22474
|
9.813x10-5
|
2.35928
1.22474
|
9.813x10-5
|
2.33860
1.22474
|
9.813x10-5
|
|
932.400
|
0.115324
|
2351.26
2349.52
|
0.115324
|
2345.98
2344.25
|
0.115324
|
2329.97
2328.23
|
0.115324
|
|
968.1219
|
0.119742
|
2441.30
2439.57
|
0.119742
|
2435.83
2434.09
|
0.119742
|
2419.20
2417.47
|
0.119742
|
|
1597.50
|
0.197587
|
4027.84
4026.11
|
0.197587
|
4018.80
4017.07
|
0.197587
|
3991.36
3989.63
|
0.197587
|
|
1626.2747
|
0.201146
|
4100.37
4098.64
|
0.201146
|
4091.17
4089.44
|
0.201146
|
4063.24
4061.51
|
0.201146
|
|
1627.3724
|
0.201282
|
4103.14
4101.41
|
0.201282
|
4093.93
4092.20
|
0.201282
|
4065.98
4064.25
|
0.201282
|
|
1882.1775
|
0.232798
|
4745.45
4743.72
|
0.232798
|
4734.80
4733.07
|
0.232798
|
4702.48
4700.74
|
0.232798
|
|
2384.200
|
0.294890
|
6010.95
6009.22
|
0.294890
|
5997.46
5995.72
|
0.294890
|
5956.51
5954.78
|
0.294890
|
|
2540.5243
|
0.314226
|
6405.01
6403.28
|
0.314226
|
6390.63
6388.90
|
0.314226
|
6347.00
6345.27
|
0.314226
|
|
2586.1286
|
0.319866
|
6519.97
6518.24
|
0.319866
|
6505.34
6503.60
|
0.319866
|
6460.92
6459.19
|
0.319866
|
|
2895.5219
|
0.358133
|
7299.89
7298.16
|
0.358133
|
7283.50
7281.77
|
0.358133
|
7233.77
7232.04
|
0.358133
|
|
3189.40
|
0.394482
|
8040.70
8038.96
|
0.394482
|
8022.65
8020.91
|
0.394482
|
7967.87
7966.14
|
0.394482
|
|
3215.90
|
0.397760
|
8107.50
8105.76
|
0.397760
|
8089.30
8087.56
|
0.397760
|
8034.07
8032.33
|
0.397760
|
|
3217.5792
|
0.397967
|
8111.73
8110.00
|
0.397967
|
8093.52
8091.79
|
0.397967
|
8038.26
8036.53
|
0.397967
|
|
3240.1630
|
0.400761
|
8168.66
8166.93
|
0.400761
|
8150.32
8148.59
|
0.400761
|
8094.67
8092.94
|
0.400761
|
|
3241.5983
|
0.400938
|
8172.28
8170.55
|
0.400938
|
8153.93
8152.20
|
0.400938
|
8098.26
8096.53
|
0.400938
|
Table 2 Calculated vibrational energies in the double potential well of the ammonia molecule
Conclusion
In previous studies, it has been achieved a simple method for the exact general solution of the radial SE for central potential well without using any approach. In this article, by using this simple procedure, the oscillations through a barrier of arbitrary form central potential for a particle in central potential well of any form was solved without any approach. It has been applied to a barrier of quadratic form potential which is found in the quadratic form of a potential wells. It seems that our results are very compatible because there is not any approach in our solutions whereas there is some approach in classical solutions. Our wave functions and our solutions can be applied to the quantum tunneling of particles through potential barriers, and in the solutions of every problem using molecular, atomic, and nuclear wave functions. In the present study, these wave functions were applied to quantum tunneling in a double square well potential which is available in the ammonia molecule. Here, triaxial ellipsoid shaped potential energy was taken, but similar calculations can be made by taking other double-well potentials. If there is a non-zero angular momentum, that is, rotational motion in a quantum system, there is always a double potential well in the excited states. Conversely, there is a rotation if there are double potential wells in a quantum system. Here, the energies can be calculated and compared with the energies found experimentally by taking another suitable double potential well or by changing the parameter. Such a study may be another research topic. Here we have shown how to calculate the double potential well.
Acknowledgments
We would like to express our sincere gratitude to Özel, Işıl, and Beril Erbil for their help in editing.
Conflicts of interest
The author declares there is no conflict of interest.
References

©2024 Erbil, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.


