Open Access Journal of
eISSN: 2575-9086


Case Report Volume 2 Issue 4
1MVZ Residen of Neurology, Peques Hospital, Postgraduate Student, Medical Research Unit in Immunology, Pediatric Hospital, National Medical Center SXXI, IMSS; Mexico City
1MVZ Residen of Neurology, Peques Hospital, Postgraduate Student, Medical Research Unit in Immunology, Pediatric Hospital, National Medical Center SXXI, IMSS; México City
2MVZ Department of the Peques Veterinary Hospital, Director of the Neurology, Mexico
2MVZ Department of the Peques Veterinary Hospital, Director of the Neurology, Mexico
3MVZ in Medicine of Dogs and Cats, Director of the Dr. Cedillo Clinic, Mexico
3MVZ in Medicine of Dogs and Cats, Director of the Dr. Cedillo Clinic, Mexico
4MVZ Director of Hospital Imagen Avenida Sur 24 No. 54 Colonia Agr
5MVZ Dog and cat medicine, DIPL. ACVR, Certified Radiologist, Mexico
6MVZ MC Department of Biological Sciences, Mexico
7Postgraduate Student, Medical Research Unit in Immunology, Pediatric Hospital, National Medical Center SXXI, IMSS; Mexico City
8Medical Research Unit in Immunology, Pediatric Hospital, National Medical Center SXXI, IMSS; Mexico CityMedical Mexico
Correspondence: Jose Alberto Toscano Zapien, MVZ. Resident of Neurology, Hospital Peques; Postgraduate Student Medical Unit in Immunology, Pediatric Hospital, National Medical Center SXXI, IMSS; Mexico City, Mexico
Received: June 26, 2018 | Published: August 10, 2018
Citation: Toscano-Zapien JA, Morales-Castro H, Cedillo-Sánchez H, et al. Necrotizing meningoencephalitis (MEN) or pug encephalitis report of a case. Open Access J Sci. 2018;2(4):282-285. DOI: 10.15406/oajs.2018.02.00087
This disease was initially described in dogs of the pug breed (for that reason it has been denominated for a long time as pug encephalitis) but later similar cases have been described in dogs of breed Bichon maltes, Chihuahua, Yorkshire Terrier, Pekingese, French Bulldog, Shi -Tzu and Lhasa-Apso. The cause is unknown although in some articles this type of meningoencephalitis is related to viral infections such as Herpesvirus Type I or canine distemper and it is believed that they could play an important role in the onset of MEN. On the other hand in some Pug affected MEN have been isolated from CSF auto-antibodies against astrocytes. As in the rest of the meningoencephalitis of unknown origin, treatment is based on the administration of corticosteroids and other immunosuppressant’s frequently associated with anticonvulsants because many of these animals have seizures.
Keywords: pug encephalitis, viral infections, CSF auto-antibodies against astrocytes, large groups, dogs, meninges, paresis and tremors, cranial nerves, seizures, vascular processes, syndrome or neoplasms,
The term meningoencephalitis refers to the inflammation of the brain parenchyma (encephalon) and the membranes that cover it (meninges). Given the close relationship between both structures, inflammation usually affects both regions and even the spinal cord, producing myelomeningoencephalitis.1,2 The causes of meningoencephalitis in dogs include two large groups: those that occur as a result of the action of an infectious agent (virus, bacteria, fungi or protozoa) and those that occur as a consequence of an exaggerated immune response and in which there is no evidence of infection by any microorganism and that sometimes have characteristics similar to certain neoplastic processes such as lymphomas, within the central nervous system.1 As in other pathologies of the central nervous system (CNS), clinical signs depend on the location of the lesion. In most cases the symptoms appear acutely or subacute, are progressive and indicate a multifocal or diffuse location of the process.2 Deficits often occur in cranial nerves, seizures, paresis and tremors. Other differential diagnoses that should be included in the presence of multifocal alterations and progressive course are metabolic, nutritional and toxic causes, multiple vascular processes and degenerative diseases such as cognitive deficit syndrome or neoplasms.2
Inflammatory processes of the CNS are frequently associated with systemic alterations such as cervical pain, fever, apathy, anorexia and hyperesthesia, the state of consciousness can be affected may be state of stupor or comatose state. Of special importance in the physical examination of these patients is ophthalmoscopic examination, since in many cases chorioretinitis or edema of the optic disc can be observed.2 Other systemic alterations that can be observed are joint or muscular pain, especially frequent in some meningoencephalitis types such as the syndrome of meningitis arteritis that responds to steroids, which can be accompanied by joint alterations.3 The presumptive diagnosis of these diseases is based on the combination of a compatible clinical history, the findings in complementary tests, mainly in the analysis of cerebrospinal fluid (CSF) and the results of diagnostic imaging tests, especially magnetic resonance imaging (MRI).).4 Many of these diseases do not present with a systemic inflammatory response and, therefore, the blood count often does not show alterations. The findings in the CSF analysis are not specific since there are many processes such as vascular accidents, traumatisms (including herniated discs) and neoplasms that may occur with abnormal CSF counts.5 MRI or tomography (CT) images are not definitive since the inflammatory lesions can resemble neoplastic or even vascular lesions. It is for this reason that the definitive diagnosis of inflammatory processes of unknown origin of the CNS is, in most cases, anatomopathological. In addition, since the changes found in the diagnostic imaging tests do not allow to identify the cause of the inflammatory process, the realization of serology or titration against infectious agents in the CSF is essential to rule out the presence of pathogens as a cause of the disease.6
Necrotizing meningoencephalitis (MEN)
This disease was initially described in dogs of the pug breed (for that reason it has been denominated for a long time as pug encephalitis) but later similar cases have been described in dogs of breed Bichon maltes, Chihuahua, Yorkshire Terrier, Pekingese, French Bulldog, Shi -Tzu and Lhasa-Apso.6–10 The cause is unknown although in some articles this type of meningoencephalitis is related to viral infections such as Herpesvirus Type I or canine distemper and it is believed that they could play an important role in the onset of MEN. On the other hand in some affected Pug of MEN have been isolated from the CSF auto-antibodies against astrocytes.11 Studies have been conducted in dogs suffering from necrotizing meningoencephalitis; the sample that is collected is cerebrospinal fluid (CSF), in which an amount of antibodies against the Fibrillary Acid Glial (GFAP) protein has been demonstrated as a specific molecular marker of this disease. A study shows that patients decrease the amount of antibodies against GFAP when they undergo immunosuppressive therapies.12 The analysis of CSF reveals a predominance of lymphocytes, histiocytes and plasma cells and a significant concentration of proteins. The visible lesions on MRI are areas of asymmetric necrosis that affect both cerebral hemispheres. The lesions are irregular, localized in both gray matter and white matter, hyperintense in the T2-weighted sequences and iso-hypointense in the T1-weighted sequences, including different degrees of parenchymal and meningeal enhancement.
Other possible findings include asymmetry of the lateral ventricles, deviation of the midline, cerebral herniation and areas of hyperintensity located in the hippocampus and pyriform lobes in the sequences weighted in T2.13,14 The changes observed in CT consist of the presence of irregular areas of hypoattenuation inside the cerebral parenchyma compatible with areas of necrosis that are frequently located in both cerebral hemispheres, although they can also be observed in other regions such as the thalamus. These lesions are usually bilateral but asymmetrical. Occasionally perilesional contrast uptake can be observed. Fifteen MEN is characterized by producing cavitated lesions in the cerebral parenchyma and loss of normal delimitation between white matter and gray matter. These areas may appear edematous instead of cavitated. The histological examination of the affected tissue demonstrates a no suppurative meningoencephalitis with extensive necrosis that affects leptomeninges and white and gray matter.1–16 The inflammatory changes are more extensive in the white matter, which is related to the macroscopic changes observed. Unlike what happens in necrotizing leukoencephalitis, the lesions do not affect the cerebellum, brainstem or spinal cord.1–16
As in the rest of the meningoencephalitis of unknown origin, treatment is based on the administration of corticosteroids and other immunosuppressant frequently associated with anticonvulsants because many of these animals have seizures. Interestingly, the administration of anticonvulsants was the only drug associated with greater survival in one study.7 As a general rule, clinical symptoms progress rapidly and the result is fatal, since most dogs die or are euthanized between1–6 months after the onset of symptoms.7 Methods to prevent the risk of this disease. Genetic tests offered: PDE, PKDef susceptibility to Pug dog encephalitis (PDE). Approximately 1.2% of Pug dogs die of necrotizing meningoencephalitis (NME), also known as Pug dog encephalitis (PDE). NME is an inflammatory disease of the central nervous system that is usually progressive and fatal. Symptoms of NME include seizures, depression, ataxia, abnormal gait, and blindness.17 Fawn-colored female Pug Dogs less than 7 years of age are more likely to develop NME than older individuals, even males. and no beige color.18 Recent research has revealed that NME susceptibility is associated with the dog leukocyte antigen (DLA) region of chromosome 12 of the dog.19 The association is in the region containing the class II DLA genes. Dogs that have two identical copies of associated NME markers in this region have an observed risk (OR) of 12.75% for NME in their lifetime, compared to Pugs that only have one or no copies of these markers (OR 0- 1.08)
The results of these genetic tests can be reported as follows:
(N / N): There are no copies of the markers associated with NME (homozygous for normality). These dogs have a low risk of developing NME. (N/S): of the markers associated with NME (heterozygotes for susceptibility). These dogs have a low risk of developing NME. (S/S) copies of the markers associated with NME susceptibility. These dogs are 12.75 times more likely to develop NME in their life.
This is not a diagnostic test for NME in Pug dogs or for the disease or risk of NME in other breeds. The test is only to determine the risk of developing NME in Pug dogs and to select matings that will produce puppies with lower risk (N / N, N / S). Although a significant proportion (11%) of Pug dogs is S/S, only about 1 in 8 of this group will develop NME during their life time. In addition, breeders are advised not to reproduce the S genotype, because 40% of Pug dogs have the S genotype in a heterozygous state (N/S=29%) or homozygous (S/S=11%). The elimination of the S genotype will lead to a considerable loss of genetic diversity. Therefore, breeders should carefully select matings that do not produce S/S puppies. The report for NME includes DNA types for a panel of 8 markers selected from the canine kinship panel of the International Society for Animal Genetics (ISAG). These markers provide individual identification for each sample analyzed.20
A patient of the Pug race named "SPIKE" was presented to the Veterinary Hospital "Peques", who is described in his review as a male of 3 years and 4 months of age with a clinical history of seizure problems. The owner mentioned that the animal was staggering when he was younger, and that he presented only one seizure when he was two months old but that he never presented it again until he was 3 years old. During the anamnesis the owner reports that he has been taking vitamins B and E, also with gabapentin. At the general physical examination no alterations were observed, blood chemistry studies and general urinalysis were performed, which did not have any alteration. At the neurological examination the patient's state of consciousness was abnormal, he was in a state of stupor since he presented loss of orientation blindness and ataxia, the patient had delayed proprioception of the four limbs. It is decided to talk with the owner and discuss the case of spike, he is told that the patient has alterations of two diseases that were suspected, the first disease that was suspected was idiopathic epilepsy, the second disease was a meningoencephalitis of origin unknown. The owner is offered the option of taking Cerebrospinal Fluid (CSF) for the study of it, the owner does not allow obtaining the CSF. It is proposed to perform an MRI to arrive at a diagnosis that allows to implement a therapeutic plan and stabilize the patient, the owner allows the study, before ordering to perform the MRI study the patient undergoes a therapy of phenobarbital and bromide of potassium, to send it more stable to the study of image, it is proposed to keep the animal for a week with these drugs and after that week submits to the image study. The result of the interpretation of the magnetic resonance is the following: Definition between gray and white substance is abnormal. Several cavitary lesions filled with cerebrospinal fluid are observed, located in the temporal and frontal lobes bilaterally and observed in the FLAIR sequences. The lateral ventricles are dilated asymmetrically. The cortical tissue is atrophied and with an increase in intensity T2 creating a diffuse hyperintensity. There are no areas of magnetic susceptibility that suggest hemorrhage or bruising. Normal caudal fossa and cerebellum. The tympanic bullae are normal. There is no enlargement of regional lymph nodes. Symmetrical muscle groups. Nasal cavity, eyeballs, nasopharynx, normal larynx (Figure 1 A & B).
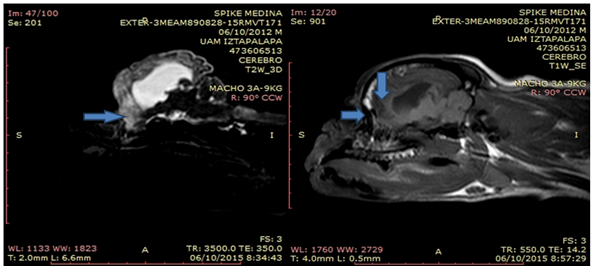
Contrast
Contrast medium is maintained in the intravascular space. There are no areas of reinforcement with contrast medium.
Interpretation
Findings compatible with Pug encephalitis (Necrotizing Meningoencephalitis). Anticonvulsant, anti-inflammatory steroid and immunomodulation are recommended. Reserved forecast. Non-obstructive hydrocephalus. The interpretation was in charge of the MVZ Esp Daniel Rodríguez Arroyo. According to the result obtained in the magnetic resonance, a therapeutic plan was implemented. The therapeutic plan that was implemented was the following: Alin (Dexamethasone) Tablets Laboratorio Chinoin. The dose of Dexamethasone was .25mg / kg BID for one week, Fenabott (Phenobarbital) dose 2mg/kg BID and Potassium Bromide dose 30mg/kg BID was also sent a gastric protective Omeprazole capsules of 20mg CID. Dexamethasone after one week was changed to prednisone at a dose of 0.25mg/kg CID. Prednisone was maintained for 2 months in that dose and then spaced every 72 hours, the therapeutic plan for seizures is maintained until now. The patient presented an improvement at 3 days with the established protocol, the owner commented that he has not presented any seizure event and he has not staggered, that the patient has eaten, urinated and defecated in a normal way, to the patient's neurological examination still presents delayed proprioception of the four members. Nine months later the patient returns to the hospital for a neurological review, according to the data reported by the owner spike has not presented any convulsive episode for six months three weeks, his state of consciousness is normal is no longer a state of stupor, the gait and posture was corrected.
CSF analysis is the most accurate antemortem method to confirm the existence of an inflammatory process of the CNS. However, this technique is nonspecific (it should be noted that a large number of non-inflammatory pathologies of the nervous system can lead to a high cell count in the CSF analysis). Due to this non-specificity, the study of patients with suspected CNS inflammatory pathology usually also requires the study by means of advanced imaging tests. On MRI or CT, focal, multiple or diffuse lesions in the cerebral parenchyma can be seen, associated or not with meningeal enhancement after administration of contrast. As in the rest of the meningoencephalitis of unknown origin, treatment is based on the administration of corticosteroids and other immunosuppressants frequently associated with anticonvulsants because many of these animals have seizures. Interestingly, the administration of anticonvulsants was the only drug associated with greater survival in one study.7 Genetic tests offered: PDE, PKDef susceptibility to Pug dog encephalitis (PDE). Approximately 1.2% of Pug dogs die of necrotizing meningoencephalitis (NME), also known as Pug dog encephalitis (PDE). NME is an inflammatory disease of the central nervous system that is usually progressive and fatal. Symptoms of NME include seizures, depression, ataxia, abnormal gait, and blindness.17 Fawn-colored female Pug Dogs less than 7 years of age are more likely to develop NME than older individuals, even males and no beige color.18 Recent research has revealed that NME susceptibility is associated with the dog's leukocyte antigen (DLA) region of chromosome 12 of the dog.19 Studies have been conducted in dogs suffering from necrotizing meningoencephalitis, the sample that is collected is cerebrospinal fluid (CSF), in which an amount of antibodies against the Fibrillary Acid Glial protein (GFAP) has been demonstrated as a specific molecular marker of this disease. A study shows that patients decrease the amount of antibodies against GFAP when they undergo immunosuppressive therapies.12
Astrocyte dysfunctiton was reported to induce disturbances in the CNS, since astrocytes modulate the extracellular concentrations of excitatory amino acids such as glutamate.21,22 Exces sive accumulation of glutamate in the extracellular space induces neuronal cell death by the excitatory neurotoxicity.23 In conclusion, GFAP is one of common autoantigens in canine NME, and the anti-GFAP autoantibodies in the CSF may be closely related to the pathogenesis and/or pathophysiological states of NME, although further studies are necessary to clarify the underlying pathological mechanisms. It must be taken into account that this is not a diagnostic test for NME in Pug dogs or for the disease or risk of NME in other breeds. The test is only to determine the risk of developing NME in Pug dogs and to select matings that will produce puppies with lower risk (N / N, N / S). Although a significant proportion (11%) of Pug dogs is S/S, only about 1 in 8 of this group will develop NME during their lifetime. In addition, breeders are advised not to reproduce the S genotype, because 40% of Pug dogs have the S genotype in a heterozygous state (N/S=29%) or homozygous (S/S=11%). The elimination of the S genotype will lead to a considerable loss of genetic diversity. Therefore, breeders should carefully select matings that do not produce S / S puppies. The report for NME includes DNA types for a panel of 8 markers selected from the canine kinship panel of the International Society for Animal Genetics (ISAG). These markers provide individual identification for each sample analyzed.20
None.
The author declares there is no conflict of interest.

©2018 Toscano-Zapien, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.