Open Access Journal of
eISSN: 2575-9086


Research Article Volume 2 Issue 1
1Faculty of Applied Sciences and Humanities, Invertis University, India
2Department of Zoology, IFTM University, India
Correspondence: Fiza Khan, Department of Zoology, IFTM University, India
Received: December 07, 2017 | Published: January 25, 2018
Citation: Mazid M, Khan F. Application of plant derived pesticides to enhanced castor productivity and yield attributes. Open Access J Sci. 2018;2(1):42-49. DOI: 10.15406/oajs.2018.02.00042
Castor is well known as good rotational crop and have achieved important place in the Indian cropping system to buildup sustainable agriculture. Castor production has multiple roles in the traditional farming systems of India. Insects are regarded as the most successful group within the Animal Kingdom, over 80% of all living beings are insects. They are man’s chief competitors on earth and to some extent his benefactors. To control any pest it is essential to have a correct idea of the insect pest identification, its biology, distribution, food range, damaging stage, mode of damage and the nutritional ecology. India is the world’s largest producer of castor seed and dominates the international castor oil trade. The chemical groups, conventionally in use today are synthetic pyrethroids, organophosphates and carbamates. The impact of synthetic pesticides on beneficial arthropods and the human health risks by exposure to these chemicals are issues of growing concerns. In perspective of the damage caused by such insects, the objectives of the present research were to assess the efficacy of two insecticides belonging to two different categories on mortality, malformation, longevity, pre-oviposition, oviposition, post-oviposition, fecundity and fertility of two lepidopterous polyphagous pest’s viz., Euproctis lunata and Spodoptera litura. Topical Application of Sublethal Concentrations of Spinosad affects the mortality, moulting and longevity, fecundity and fertility, pre-oviposition, oviposition and post-oviposition period. Spinosad and deltamethrin can be recommended for the control of Spodoptera litura. The recommended concentrations may be regarded as safe for spray operator because the application of high concentrations put the spray operator at greater risk, lead to residue hazards or prove uneconomic.
Keywords: agriculture, castor, insect, larvae, pesticide, productivity
Today, India ranks second worldwide in farm output. It has a large and diverse agriculture and is one of the world’s leading producers as well as a major consumer with an expanding population to feed. Insects are regarded as the most successful group within the Animal Kingdom, over 80% of all living beings are insects. It is very nutritive and oil used as an adjunct to starchy diets. It is given as preventive diet to CVD patients because of its rich source of Ca, P, Mg, Fe, and K content. They are man’s chief competitors on earth and to some extent his benefactors. In spite of the numerous advance made by man in evolving newer and deadlier weapons to fight the war against insects, he has not succeeded in controlling the thousands of serious pests which damages his food and other agricultural products.1,2 The widely used method for the control of a pest is through different insecticides or through bio-pesticides. The problems caused by synthetic pesticides and their residues have increased the need for effective, bio-degradable insecticides with greater selectivity. It is clear that the excessive use of insecticides in agriculture is a serious cause of concern, therefore, use of bio-pesticides considered as safer substitute. Many of the growers are using different types of preparations based on plants and other organic substances, which are known to have insecticidal properties.3,4 Most of the plants used in the preparations are locally available and hence farmers will be able to prepare the formulations themselves and apply to the plants. Many plants, microbes and their secondary metabolites are known to have various insecticidal properties against different species of insect. The plant products that are traditionally used and produced by the farmers in developing countries appear to be safe and promising.
The impact of synthetic pesticides on beneficial arthropods and the human health risks by exposure to these chemicals are issues of growing concerns. This has prompted the development of new compounds, an example is Spinosad. Spinosad, a macrocyclic compounds produced by actinomycete Saccharopolyspora spinosa. Mertz and Yao isolated from a jamician soil sample.5 Spinosad, on the other hand, is a bacterial waste product produced by fermentation on a nutrient food source used by the one particular bacterium (Saccharopolyspora spinosa). As these products are created biosynthetically, it has been classified as; an organic substance by the USDA National Organic Standards Board.6 It is also OMRI Listed for use in organic production. Until spinosad (pronounced spin-OH-sid) was discovered, one of the only organically acceptable insecticides was BT (Bacillus thuringensis). Spinosad acts as a stomach and contact poison and degrades rapidly in the environment.7,8 Sunlight and soil microbes break it down into carbon, hydrogen, oxygen and nitrogen. It has little toxicity to birds and mammals.9 It can be used on outdoor ornamentals, lawns, vegetables and fruit trees, to control caterpillars, thrips, leafminers, borers, fruit flies, spidermites, aphids, and more. It is effective as a protectant of stored grains10–12 and as a residual application to flooring surfaces13,14 (Figure 1).
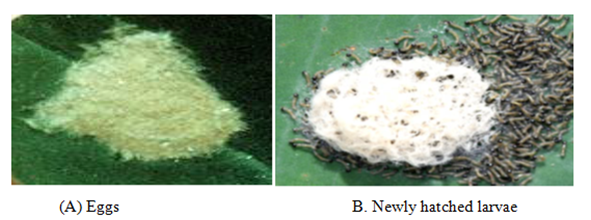
Castor is an important crop grown in tropical world. Castor cake is used in agriculture as organic manure due to its high nitrogen content. The world castor seed production has fluctuated from a low of 937,000tones to a high of 1,488,000 tones. It contributes about 62% of the world production and ranks first. The top producer of castor seed in India is Gujarat, with 86percent share, followed by Rajasthan and Andhra Pradesh. Among the several factors that contribute to low productivity of castor, the insect pests constitute the major factor. Crop suffers heavily due to attack of various pests, which reduces their yield. Oriental leaf worm moth Spodoptera litura is a Noctuid moth which is considered as a serious but sporadic agricultural insect pest causes economic losses of crops from 25.8-100%15–17 based on crop stage and its infestation level in the field. It is also known as the Cluster caterpillar, Cotton leaf worm, Tobacco cutworm, and Tropical armyworm. It is one of the most economically important insect pests in many countries including India, Japan, China, and other countries of Southeast Asia. After emergence between 2 and 5days, female lays 1000-2000eggs on the lower surface of host plant. The egg is round and dirty white. Eggs hatched between 4-5days. The newly emerged larva is whitish and then turns yellow green an hour after with a pattern of red, yellow, and green lines from the head to the anal region. As the larva grew bigger, the body turns brown with 3 thin yellow lines down the back. The body of the newly emerged larva is cylindrical, head size is wider than. In perspective of the damage caused by such insects, the objectives of the present research were to assess the efficacy of two insecticides belonging to two different categories on mortality, malformation, longevity, pre-oviposition, oviposition, post-oviposition, fecundity and fertility of one lepidopterouspolyphagous pest, Spodoptera litura (Figure 2).
Adults of Spodoptera litura were collected in night near lamp posts in the Aligarh Muslim Campus during August to October. Several pairs of adults were kept in separate circular rearing jars made of glass measuring 8”×4”. The bottom of the jars was fitted with wet and sterilized sand which was about two inches in thickness. The tops of the jars were covered with muslin cloth which was tightly fixed by means of rubber bands. All the rearing jars were kept at 28±1°C temperature and 70-80% relative humidity. The moths were fed on saturated glucose solution. For this purpose, a piece of sterilized cotton wool was wrapped around a micro-slide and it was soaked with fresh sugar solution. This soaked slide was obliquely placed against the jar wall. A freshly soaked slide was daily replaced. The females laid eggs on the white paper strips that were placed in the rearing jars. The paper strips along with eggs were placed in fresh jars for hatching. Following the hatching of the eggs, young larvae were given tender castor leaves for feeding. The jars having larval stages were daily cleaned and fresh leaves were provided daily. When the larvae reached to late 6th instar they were transferred to separate jars containing 3-4inch thick layer of slightly damp sand for pupation.
The larvae went 2-3inch down in the sand and pupated. After two days of pupation, the pupae were taken out from the sand, cleaned with cotton and kept in separate jars for emergence. As soon as the moths emerged they were transferred to other rearing jars to maintain a large population of all stages throughout the year at the controlled condition. From this stock culture, newly moulted 5th instar larvae were sorted out and kept in separate breeding jars for further experimental purposes. In the present study 24hrs old 5th instar larvae were used for the experiments. The samples of newly moulted 4th instar larvae Spodoptera litura between 10:00am and 12:00 noon each day were isolated and maintained age-wise. Subsequently, fresh moulted 5th instar larvae were treated with different concentrations of spinosad and deltamethrin. Likewise, the 4th instar larvae of Spodoptera litura were also sorted out from the random culture and maintained in separate rearing jars. As soon as they moulted to 5th instar which can be visually ascertained by the size, head capsule and exuviae they were treated with varying concentrations.
Procedure
These sub lethal concentrations of each insecticide were used for topical application on the 5th instar larvae of Spodoptera litura. The effect of these chemicals were observed on mortality, malformation, fecundity, fertility and longevity by using four sub-lethal concentrations of each insecticide at the above said controlled conditions. For each concentration of diluted insecticide only 1µl was applied topically on the thoracic pleuron of individual 5th instar larvae of both insects by 26-guage needle attached to a tuberculin syringe fitted into a manually operated micro applicator. At the time of application the age of the experimental insect was one day. For each concentration 100 individual larvae were used. Two sets of control consisting of same number of larvae of same age were also maintained parallel with each set of experiment at the same controlled conditions to compare the results. One set were treated with 1µl acetone solution (solvent) and regarded as control-I, whereas the second set also having the same number of untreated larvae of same age was regarded as control-II, to compare the acetone treated larvae. When females subsequently emerged from the survived treated larvae they were paired with the males emerged from the untreated larvae of corresponding age from control-II.18 The number of eggs laid by each females and fertility were recorded. The un-hatched eggs were also counted. Similar observations were also made in controls consisting of the males and females emerged from acetone-treated as well as untreated larvae. The data on mortality, malformation, longevity, hatching of Spodoptera litura affected by different concentration of spinosad and deltamethrin were recorded for each replicates and mean value of three such replicates of each treatment was calculated.
Effect of spinosad
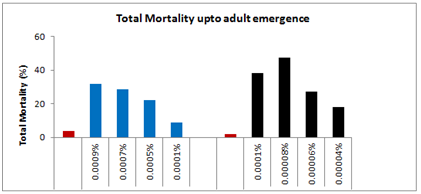
Mortality, moulting and longevity: The first set which consisting of 25 larvae of 24 hrs old 5th instar was topically treated with 1µl of acetone solution only, the initial mortality within 24 hrs was nil, however the total larval mortality up to adult emergence was 4%. The larvae that survived after the treatment were quite active, healthy, showing normal feeding and moulted to adult successfully (Plate-II). In the second set, the topical application of the lowest selected sub-lethal concentration of spinosad (0.0001%) on 5th instar larvae caused only 3.33% larval mortality within the same instar and later 5.67% up to adult emergence leading to a total loss of 09% which is only 05% more as compared to control-I. The longevity of all the surviving treated 5th instar larvae was 28.57% more, whereas, the survival duration of the next instar was 18.03% more as compared to control. Likewise, the adult males which emerged from the survived treated larvae lived 37.36% more as compared to control, whereas the longevity of affected females that also emerged from the survived treated larvae was enhanced by 29.38%as compared to control-I.
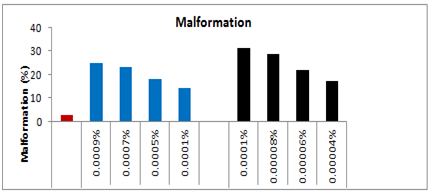
In the third set, topical application of 0.0005% spinosad resulted 8% larval loss within the same instar, whereas 6% larvae died during the next larval stage and 03% died due incomplete larval-pupal transformation and 05% could not emerged successfully from the pupae and succumbed at during pupal-adult emergence. The total loss up to adult emergence was 22% which was 18% more than the control-I. Out of the total adults, 18% had malformed wings. The longevity of the survived 5th and 6th instar larvae was enhanced by 39.90% and 35.62% respectively, whereas, the survival duration of adult males and adult females was increased by 46% and 33.33% respectively as compared to control-I (Figure 3). In the fourth set of 25 larvae treated with 0.0007% concentration of spinosad, the immediate larval mortality within 24 hrs was 14%, however, 7.33% larvae died during the ensuing instar followed by 3% during the larval-pupal transformation and 4.33% during the adult emergence, leading to a total mortality of 28.66% up to adult emergence. The larvae which survived the treatment were quite active like those of control. However, out of adults who emerged from the survived larvae, 23.33% had malformed wings.
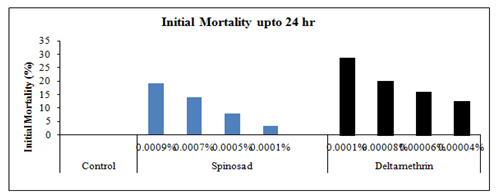
The larval duration of the survived treated 5th instar larvae was enhanced by 55.83%, whereas, the longevity of 6th instar larvae was prolonged by 48.54% as compared to control-I. In the last set of experiment when 25 larvae (5th instar) were treated topically with 0.0009% spinosad, 19.33% larvae died during the same instar (Plate-II,), whereas 5.67% larvae succumbed at next instar. Further, 02% larvae died during the next stage i.e. larval-pupal transformation could not cast off their exuvae and 05% died during the process of pupal-adult ecdysis. The total larval mortality up to adult emergence was 32%, which was 28% more than the control. Among the adults which emerged from the survived larvae, 24.66% had malformed wings or legs. The mean longevity of 5th and 6th instar larvae was prolonged by 39.9 and 40% respectively, as compared to the control (Figure 4).
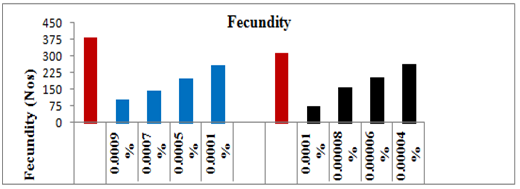
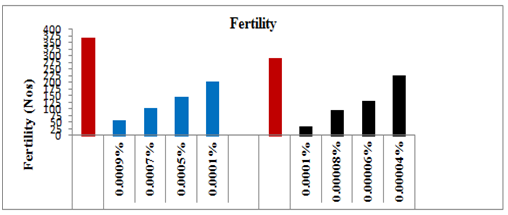
Fecundity and fertility
The adults which emerged from the survived treated larvae had no effect on their behavior and were as competitive as those of controls. The newly emerged affected males were paired with females of same age of the same set to observe fecundity and fertility. The average egg production of the females that emerged from the survived treated 5th instar larvae decreased linearly with increasing concentrations of spinosad (Y=–67.49x+489.1, R2=0.949, P≥0.001) showing a negative correlation coefficient (Figure 5). After the application of the lower selected sub lethal concentrations (0.0001 and 0.0005%) of spinosad, the average fecundity of the females reduced significantly by 32.06% (t=10.383, P≥0.05) and 48.18% (t=14.473, P≥0.05), respectively, as compared to the control. Whereas on application of the higher sub lethal concentrations, i.e. 0.0007 and 0.0009% spinosad on the larvae, the average egg production by the females dropped significantly by 62.13% (t=15.847, P≥0.05) and 72.70% (t=11.111, P≥0.05), respectively, as compared to control-I. Likewise, the fertility of the eggs laid by the affected females also decreased linearly and showed negative correlation (Y= –72.06x+464.4, R2=0.900, P≥0.001). The hatchability of the eggs after the application of lower doses dropped significantly by 21.93% (t=12.789, P≥0.05) and 26.09% (t=19.974, P≥0.05), respectively, as compared to control-I. Whereas on the application of the higher selected sub lethal doses i.e. 0.0007% and 0.0009%, the fertility of the eggs dropped significantly by 29.06% (t=13.332, P≥0.05) and 44.76% (t=14.551, P≥0.05), respectively, as compared to control-I (Figure 6).
Pre-oviposition, oviposition and post-oviposition period
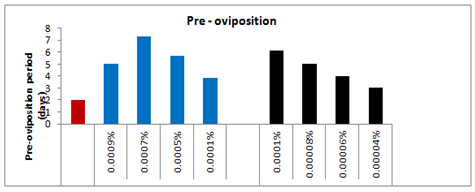
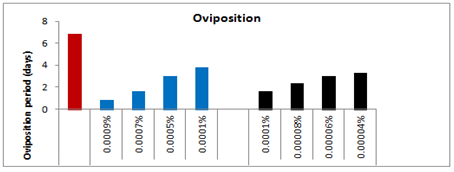
The pre-oviposition period of the affected females emerged from the treated larvae with 0.0001%, 0.0005%, 0.0007% and 0.0009% concentrations of spinosad was recorded as 3.83(t=2.200), 5.66(t=3.617), 7.33(t=4.880) and 5.00(t=1.078) days respectively as compared to 2.00days in case of control (Figure 7). Thus, the overall increase in the pre-oviposition period of the affected females was 47.78%, 64.66%, 72.71% and 60% respectively as compared to control-I. Likewise, the post-oviposition period of the affected females that emerged from the survived treated larvae with respect to the above said concentrations was 6.66(t=0.229), 6.00(t=1.000), 7.33(t=1.387) and 6.33(t=0.053) days as compared to 6.5days in case of control which was 2.4% more, 8.33% less, 11.32 more and 2.69% less more as compared to control. On the other hand the oviposition period of the affected females with respect to the above said concentrations was reduced by 43.92, 56.08, 75.69, and 87.85% respectively as compared to control and recorded as 3.83(t=3.928), 3.00(t=8.693), 1.66(t=11.717) and 0.83(t=5.196) days respectively as compared to control i.e. 6.83days.
Effect of deltamethrin
Mortality, moulting and longevity: The first set which consisting of 25 larvae, the topical application of 1µl acetone solution did not resulted any initial mortality within 24hrs, however the total larval mortality up to adult emergence was 2% which was 03% less than that of untreated larvae (control-II) (Figure 8). The larvae that survived after the treatment were quite active, healthy, showing normal feeding and moulted to adult successfully (Plate-II,). In the second set, the topical application of the lowest selected sub lethal concentration of deltamethrin (0.00004%) on 5th instar larvae cause 12.66% initial larval loss within 24 hrs, followed by 03% larval mortality during the next instar and only 02.34% loss during larval-pupal transformation, thus resulting of 18% larval loss up to adult emergence. The longevity of all the surviving treated 5th larvae was increased by 11.66%, whereas, the survival duration of next instar was enhanced by 9.91% (Figure 9). The survivability of adult males was hiked by 17.58%, however, the survival duration of adult females was only increased by 4.76% as compared to control-I.
In the third set, topical application of 0.00006% Deltamethrin resulted 16% mortality within the same instar, whereas 9% larvae died during the next larval stage and 2.33% larvae died due to incomplete pupal-adult emergence. The total loss up to adult emergence was 25.33% more than the control-I. The adults which emerged from the survived treated larvae had 22% malformation in their wings or legs. The larval duration of the treated 5th instar was enhanced by 28.57%, whereas, the survival duration of 6th instar was increased by 25%. Likewise, the longevity of the adult males as well as adults females also increased by 38.97 and 10.39% respectively as compared to control-I. In the fourth set of 25 larvae that topically treated with 0.00008% concentration of deltamethrin, the immediate mortality was 20% during 24hrs followed by 13.33% during the next instar. Out of the survived larvae again 8.3% lost their live during pupal formation 5.7% could emerge as adult, resulting thereby a total loss of 47.33% which was 45.33% more as compared to control-I (Figure 10).
Most of the adults were quit active like those of control, but 28.66% adults developed malformation either in their wings or legs. The longevity of the larvae that survived the treatment was increased by 37.5%, whereas, the longevity of the 6th instar was enhanced by 37.88%. Similarly the survival duration of adult males was increased by 40.50% and survival duration of adult females was enhanced by 24.01% as compared to control-I. In the final fifth set of experiment when 25 larvae (5th instar) were treated topically with 0.0001% of deltamethrin, 28.66% larvae died during the same instar, whereas 2.34% larvae succumbed at next instar moulting. Further, 03% larvae died during the 6th instar and 04 could not cast off their exuvae and died during the process of larval-adult ecdysis. The total larval mortality up to adult emergence was 38%, which was 36% more than the control-l. Among the adults which emerged from the survived larvae, 31.33% had malformed wings or legs. The mean longevity of 5th and 6th instar larvae was prolonged by 48.24 and 48.54% respectively, as compared to the control. Whereas, the mean longevity of adult males and females which emerged from the survived treated larvae was increased by 43.38 and 31.03% respectively, than the control-I.
Fecundity and Fertility
The adults which emerged from the survived treated larvae had no effect on their behavior and were as competitive as those of controls. The newly emerged affected males were paired with females of same age of the same set to observe fecundity and fertility (Figure 11). The average egg production of the females that emerged from the survived treated 5th instar larvae decreased linearly with increasing concentrations of spinosad (Y= – 58.8x+438.4, R2=0.987, P≥0.001) showing a negative correlation coefficient. After the application of the lower selected sub lethal concentrations (0.00004 and 0.00006%) of deltamethrin, the average fecundity of the females reduced significantly by 16.16% (t=10.384, P≥0.05) and 36.11% (t=11.371, P≥0.05), respectively, as compared to the control-I. Whereas on application of the higher sub lethal concentrations, i.e. 0.00008 and 0.0001% deltamethrin on the larvae, the average egg production by the females dropped significantly by 49.10% (t=7.282, P≥0.05) and 76.66% (t=9.512, P≥0.05), respectively, as compared to control-I. Likewise, the fertility of the eggs laid by the affected females also decreased linearly and showed negative correlation (Y= –64.36x + 412.9, R2=0.979, P≥0.001). The hatchability of the eggs after the application of 0.00004, 0.00006 and 0.00008% concentration was dropped significantly by 13.85% (t=11.839, P≥0.05), 36.03% (t=16.290, P≥0.05) and 40.04% (t=15.829, P≥0.05) respectively, as compared to control-I. Whereas on the application of the higher selected sub lethal doses i.e. 0.0001%, the fertility of the eggs dropped by 53.84% (t=16.783, P≥0.05), respectively, as compared to control-I.
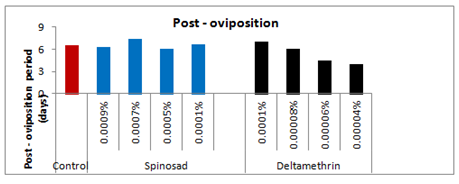
Pre-oviposition, oviposition and post-oviposition period
Following the topical application of 0.00004, 0.00006, 0.00008, and 0.0001%, concentrations of deltamethrin on 5th larval instar of Spodoptera litura, the pre-oviposition period of the females emerged from the survived treated larvae was significantly increased by 33.33, 50.00, 60.00 and 67.53% respectively as compared to control-I. On the other hand the oviposition period of the affected females emerged from the survived treated larvae following the topical application of the above said sub-lethal concentrations of deltamethrin was decreased by 51.24, 56.08, 65.89 and 75.69% respectively as compared to control-I. Similarly, the post-oviposition period following the exposure of 0.00004, 0.00006, and 0.00008% concentration resulted in reduction of post-oviposition period by 38.46, 30.77 and 7.92% respectively, however, the exposure of the highest selected sub-lethal concentration of deltamethrin (i.e. 0.0001%) enhanced the post-oviposition period by 7.14% as compared to control-I (Figure 12).
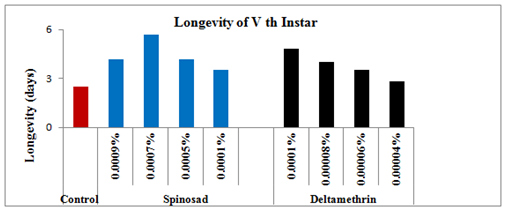
Figure 12 Longevity of 5th instar treated larvae of Spodoptera litura following topical application of Spinosad and Deltamethrin.
Insecticides are often the only effective remedy for quickly and inexpensively reducing the pest population below the economic injury levels. If in anyway crop is being damaged by pest attack, we must have to prevent it from damage by using the appropriate methods. The use of toxic chemicals and bio-pesticides for the control of pest increases tremendously during the last few decades. The toxicity of insecticides to humans and wildlife has caused much public concern and prompted the use of more target-specific chemicals. This approach has led to the development of botanical such as citrus oil, derivatives, neem-azadirachtinetc, soaps and oils, microbial insecticides such as Beauveria, Bacillus thruingensis, pheromones, natural products like spinosads, nitenpyram, imidaclopridetc, which are able to efficiently control agricultural pest species with minimum effects of natural enemies.19 Spinosad is a mixture of spinosyn A and D which are tetracyclic-macrolide secondary metabolites produced by anactinomycete, Saccharopolyspora spinosa. This compound has two unique modes of action, acting primarily on the insect nervous system at the nicotinic acetylcholine receptor and exhibiting activity at GABA receptor (Figure 13).
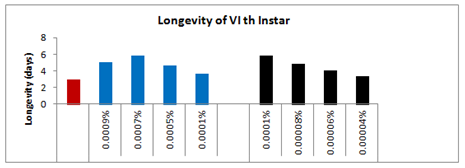
Figure 13 Longevity of 6th instar larvae emerged from 5th instar treated larvae of Spodoptera litura following topical application of Spinosad and Deltamethrin.
It is a broad spectrum natural bio-insecticide offered a new mode of action and relatively safe on natural enemies.20 Currently there are only a few cases of insect resistance to spinosad and it is not known to share cross-resistance mechanisms with any existing class of insecticide.21 On the basis of simple reciprocal crosses and backcrosses, resistance appears to be inherited as a co-dominant trait controlled by a single locus. Furthermore, they concluded that in general, spinosad has larger margins of safety for parasitoids and predators but its higher concentrations may prove lethal to certain beneficial arthropods. The efficacy of spinosad can be conserved if it is judiciously rotated with other suitable insecticides in a spray program and the maximum number of applications is restricted. Thus, it becomes inevitable to find out the most effective chemicals against particular species of insect pest which do not adversely affect the environment and are also bio-degradable. In the present investigation the efficacy of two insecticides (deltamethrin and Spinosad) belonging to two different categories (conventional and non-conventional) on one lepidopterouspolyphagous pests Spodoptera litura was studied and the results were recorded on mortality, metamorphosis, longevity, pre-oviposition period, oviposition period, post-oviposition-period, fecundity as well as fertility of the adults.
The topical application of four selected sub lethal concentrations of spinosad viz., 0.0009, 0.0007, 0.0005 and 0.0001% on 24 hrs old 5th instar larvae of Spodoptera litura gave varied mortality and increased with increasing sub lethal concentrations of spinosad viz., 0.0009, 0.0007, 0.0005 and 0.0001% on 24 hrs old 5th instar larvae of Euproctislunata and Spodopteralituragave varied mortality and increased with increasing sub lethal concentrations. The effect of the above said concentrations either on initial mortality with in 24 hour or up to adult emergence was more pronounced when the application was made on 5th instar larvae of Euproctislunata as compared to the 5th instar larvae of Spodopteralitura. The initial mortality following the topical application of the highest selected sub lethal concentration (i.e. 0.0009%) of spinosad in case of E. Lunata was 20.67% more as compared to S. Litura, whereas, the lowest selected concentration (i.e. 0.0001%) showed 11.33% more larval death in case of Spodoptera litura compared to S. Litura. Likewise the total mortality of larvae up to adult emergence following the exposure of highest (0.0009%) and lowest (0.0001%) selected concentrations of spinosad to 5th instar larvae of S. Litura. Likewise the topical application of four different selected sub lethal concentrations of deltamethrin viz., 0.00004, 0.00006, 0.00008 and 0.0001% on 24 hrs old 5th instars larvae of Spodoptera litura also gave varied mortality and increased with increasing sub lethal concentrations (Figure 14).
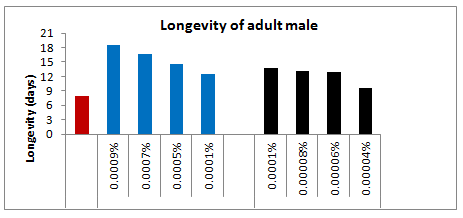
Figure 14: Longevity of adult male emerged from 5th instar treated larvae of Spodoptera litura following topical application of Spinosad and Deltamethrin.
The effect of the above said deltamethrin concentrations either on initial mortality with in 24hour or up to adult emergence was more pronounced when the application was made on 5th instar larvae of Spodoptera litura. The initial knockdown effect within 24hrs as well as the residual toxic effect up to adult emergence in case of E. Lunata following the topical application of the highest selected sub lethal concentration (i.e. 0.0001%) deltamethrin was respectively 3.33% and 9.33% high. However, if we compare the overall toxicity of both the insecticides (i.e. spinosad and deltamethrin) against the individual insect and deltamethrin prove to be good for S. Litura. The total larval mortality up to adult emergence was due to partly by direct toxicity and partly by moulting failure or malformations at the intermediate stage. The results are similar to those reported by earlier workers.22 The contact and oral toxicity of commercial formulations of spinosad and deltamethrin to adults of the crucifer flea beetle, Phyllotretacruciferae (Goeze) and showed that method of exposure had a significant effect on flea beetle mortality and feeding damage to canola seedlings. Topical treatment of flea beetles with deltamethrin or different concentrations of spinosad resulted in significantly lower mortality and higher feeding damage than exposure to treated canola cotyledons. Furthermore they reported that spinosad was more toxic by ingestion than by topical contact (Figure 15).
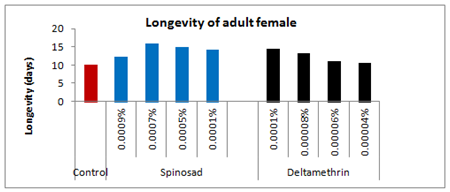
Figure 15: Longevity of adult female emerged from 5th instar treated larvae of Spodoptera litura following topical application of Spinosad and Deltamethrin.
Mortality from treated cotyledons was significantly higher with 60ppm deltamethrin than with 80 or 120ppm spinosad after 24h exposure but not after 120h exposure. Delayed mortality in the spinosad treatments did not result in high feeding damage; damage after 120h was not significantly different in the spinosad and deltamethrin treatments. Low concentrations of spinosad (40ppm) strongly inhibited feeding activity within 24h after exposure. Mortality from spinosad was higher after beetles were exposed to treated cotyledons for 120h than for 24h. Mortality from spinosad, but not deltamethrin, was significantly higher at 25°C than at 15°C. An ionic surfactant, polyethylenimine, increased the toxicity of 40ppm spinosad. On the basis of the results they suggest that spinosad has potential for use as an insecticide against crucifer flea beetles on canola.23The fecundity and fertility of the affected females of S. Litura emerged from the 5th instar nymphs treated with various selected sub lethal concentrations of both insecticides i.e. spinosad and deltamethrin decreased linearly with increasing concentrations. If we compare the efficacy of both the insecticides against Spodoptera litura then spinosad proved to be good in causing the maximum reduction in egg production, whereas, deltamethrin caused the maximum reduction in fertility of the eggs.
But when we compare the efficacy of the above said insecticides against S. Litura than deltamethrin showed the maximum reduction of egg production and egg hatching and proves to be good as compared to spinosad. Similarly, a significant reduction of fecundity was observed when Ceratitis capitata adults ingested 0.1mg/liter spinosad.24 Radiant at 5.76gram active/ HA showed 100% mortality of entire hatched egg masses of Spodoptera littorilis after the spray in the field.20 Similarly Garcia & Rembold25 recorded that the adult longevity, population growth and fecundity of Plutellaxylostella was strongly reduced when the larvae were treated with spinosad. Amer26 recorded that spinosad caused reduction in adult longevity, fecundity and fertility of P. gossypiella. Also, Mehrotra27 and Zaliznaik & Nugegod28 reported that spinosad high affected on fertility and fecundity of Bactericerca cockerelli. Egg fertility was reduced in Helicoverpazea (Boddie) when females were fed with concentrations≤1 mg (AI)/liter and then paired with untreated males.29 Singh & Vyas et al.30–35 observed the ovicidal affect of some pyrethroids which are tested against Earisvitella on okra and Spodoptera on castor and found that Decamethrin (Deltamethrin) is most affective against the eggs of Earis vitella followed by Cypermethrin and Permethrin. The effect of deltamethrin on the hatching of eggs of two strains, susceptible (S) and resistant (R), of Plutella xylostella was investigated by Ho & Goh31 and daily observations were made on the development of the embryos, hatching and larval mortality after treatment with different dosages of deltamethrin. This compound exhibited a dosage-mortality response against the S-strain eggs.
The hatching of the R-strain eggs was also affected by deltamethrin but to a lesser extent. At least 50% of the S-strain larvae that hatched from the treated eggs died within a few hours, but the larvae of the R strain were more resistant. Seedlings treated with different dosages also showed a drop in egg hatching and less damage on the leaves. Deltamethrin had no effect on embryonic development. Therefore, spinosad and deltamethrin can be recommended for the control of Spodoptera litura. The recommended concentrations may be regarded as safe for spray operator because the application of high concentrations put the spray operator at greater risk, lead to residue hazards or prove uneconomic.
I am also grateful to Dr. Khalil Khan, KVK, Chandra Sekhar Azad Agricultural University, Kanpur for his critical comments and valuable suggestions with regard to the preparation of this manuscript and ICMR, New Delhi for financial assistance.
The authors declare that there is no conflict of interest.

©2018 Mazid, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.