Open Access Journal of
eISSN: 2575-9086


Short Communication Volume 2 Issue 5
1Programa de Posgrado de Doctorado en Ciencias Biologico Agropecuarias, Universidad Autonoma de Nayarit, Mexico
2Laboratorio de Bioingenieria Costera, Universidad Autonoma de Nayarit, Tepic, Nayarit, Mexico
3Instituto Tecnologico de Mexico, Unidad Mazatlan, Sinaloa, Mexico
4Centro de Tecnologia de Alimentos, Universidad Autonoma de Nayarit, Ciudad de la Cultura Amado Nervo, Tepic, Nayarit, Mexico
Correspondence: Jesus T Ponce Palafox, Laboratorio de Bioingenieria Costera, Universidad Autonoma de Nayarit, Tepic, Nayarit, C.P. 63155, Mexico, Tel +052-3112118800, Fax +052-3112118800
Received: October 04, 2018 | Published: October 24, 2018
Citation: Carvajal-García A, Ponce-Palafox JT, Spanopoulos-Hernández M, et al. Colour and carotenoid content in different body components in Pacific red snapper (Lutjanus Peru). Open Access J Sci. 2018;2(5):379-381. DOI: 10.15406/oajs.2018.02.00101
Background: The purpose of this study was to evaluate the effects of increasing concentrations of astaxanthin (Carophyll Pink) on color and pigment content in the skin of Pacific red snapper. Wild organisms (163.0 to 176.0 g) were fed with experimental diets supplemented with 0, 25, 50 and 100 mg astaxanthin/kg (unesterified forms Carophyll Pink) for 30 days. It was found that the lowest values of L*, a*, b*, Cab* and H°ab were recorded in the wild Pacific red snapper and the higher values in AX100 treatment. The colors in the Pacific red snapper depend upon type and concentration of pigment in the skin. it was found that the application of unesterified (Carophyll Pink) in the diet improves the appearance of the Pacific red snapper giving it a salmon-colored hue (red-orange) mainly in the ventral area, in the treatments AX50 and AX100. The highest concentration of pigments was found in the dorsal > pectoral > ventral area.
Keywords: pigmentation, preference ranking test, fresh snapper
High-quality aquatic products have stimulated interest in biochemical application of carotenoids for fish pigmentation cultivated, since the color provides an added value to some fish species such as salmon, rainbow trout, tilapia and snappers among others.1 The Pacific red snapper feeds on small crustaceans mainly what gives it the red color in the skin, which is attractive in the market.2 The Pacific red snapper feeds on small crustaceans mainly3 what gives it the red color in the skin, which is attractive in the market. However, the color in the snapper is not consistent culture conditions,2 so the inclusion of pigments in the feed can be an alternative to maintain quality and market presentation snapper. The pigment most frequently found in fish is Astaxanthin (3.3 'dihydroxy-β-carotene 4,4-dione) which, with its esters, is the main pigment in the Salmon meat, giving a pink color to pinkish orange.4 Some factors that determine the degree of pigmentation of organisms are genetic aspects of the fish, state of sexual maturation and weight.5 The most commonly used pigment commercially is synthetic astaxanthin.6 The pigmentation studies in snappers are scarce. However, the inclusion of unesterified forms as Carophyll PinkTM has been studied in Australian snapper Pagrus auratus7 and considered the need for pigmentation in spotted rose snapper.8 The major aims of this study were to evaluate the effects of increasing dietary concentrations of astaxanthin (Carophyll Pink) on skin colour and pigment content in the skin of Pacific red snapper.
Wild L. peru were obtained from the wild, captured in front of La Peñita Beach, Municipality of Compostela Nayarit, Mexico (21°03'06.65"N, 105°15'58.17"O) during the spring of 2017. All fish were in a range of weight of 163.0 to 176.0 g. These fish were selected and transported at low temperature to Laboratory Coastal Bioengineering, Autonomous University of Nayarit, Nayarit, Mexico (21°29ʼ N:105°12ʼ W) for immediate colour and pigment evaluation. Four isonitrogenous and isocaloric experimental diets were formulated to contain 0% (AX0), 0.025% (AX25), 0.050% (AX50), and 0.1% (AX100) Carophyll pink TM (DSM Nutritional Products Ltd., Basel, Switzerland) containing 10% astaxanthin. The diet without astaxanthin was considered as the control diet. Tanks of 750 L were used, with a closed system and with aeration system for each tank, each with 10 organisms. They were fed three times a day and the amount of food equivalent to 2% of their biomass was supplied for 30 days. Snapper color was determined using a Minolta Chromo Meter CR 400 (Osaka, Japan) colorimeter with an 8 mm aperture and D65 illumination at a 10° angle. Measurements were taken from the Pacific red snapper on three areas dorsal, pectoral and ventral and performed in the colorimetric space9 L* (percentage of lightness), a* (red-to-green scale), b* (blue-to-yellow scale), at 25±1°C. From a* and b* values, hue angle (H°ab; overall colour) were calculated: H°ab = arctan (b*/a*) for a* and b* > 0. The chroma (Cab*; measure of % of pure hue specific to each colors maximum intensity possible or saturation): Cab*= (a*2+b*2)1/2. The amount of total carotenoids was calculated using the extinction coefficient of 2,500 for total carotenoids in petroleum ether.10 The data obtained in the reading of the spectrophotometer were used to determine the existing amount of total carotenoids.11 Colorimetric and carotenoids analysis data were analyzed by normality, independence, and homogeneity, Bartlett and Kolmogorov-Smirnov tests and one-way variance analysis (ANOVA) and Tukey’s test (P < 0.05). All tests were performed using Statistica v.5.5. (1984-2000 by StatSoft, Inc. USA.). Data were expressed as mean ± standard error.
The color pattern to the naked eye of the Pacific red snapper (Figure 1) shows a more homogeneous and salmoned color in AX100 treatment and the color of the wild snappers are smaller. It was found that the lowest values of L*, a*, b*, Cab* and H°ab were recorded in the wild Pacific red snapper and the higher values in AX100 treatment. Also a tendency was found of the values of L* to make higher in the ventral zone and a*, b*, Cab * and H°ab higher in the dorsal zone of the Pacific red snapper in all the treatments. The distribution of the carotenoids in the three body parts studied the Pacific red snapper showed that the concentration was higher in the dorsal and ventral at the bottom in all treatments (Table 2). A ventral concentration of carotenoids was determined significantly in the wild organisms with all the treatments and the highest in the AX100 treatment.
In general terms, the color values registered in this experiment were high in the Pacific red snapper, which is supported by the shown in Table 1 & Figure 1. Deposition of astaxanthin in the skin resulted in augmentation in lightness, redness, yellowness, Chroma and Hue. The inclusion of 100 mg/kg of astaxanthin was the treatment that provided the best response of the skin color of the Pacific red snapper, surpassing the control treatment (5.0%) and the wild organisms (20.9%). The experiment showed that the time of one month used for pigmentation with Carophyll Pink was adequate for all treatments and was in accordance with the saturation found in the red sea bream Pagrus major.12 The L* values (39 to 72) for the Pacific red snapper were slightly higher than those found (55 to 60) in red sea bream.13 The inclusion of Carophyll Pink in the diet significantly increased the carotenoid content in the skin of the organisms and the concentrations determined in the three zones of the Pacific red snapper were slightly higher than those recorded in red sea bream, Australian snapper and red porgy in other studies.7–14 In addition, increase in the concentration of pigments in the skin was linear with the increases of astaxanthin in the diet of Pacific red snappers, relationship that has been established for skin and muscle in rainbow trout.15 The highest carotenoid contents were observed in the dorsal area, in the treatments of AX50 and AX100. In conclusion, the colors in the Pacific red snapper depend upon type and concentration of pigment in the skin. it was found that the application of unesterified (Carophyll Pink) in the diet improves the appearance of the Pacific red snapper giving it a salmon-colored hue (red-orange) mainly in the ventral area, in the treatments AX50 and AX100. The highest concentration of pigments was found in the dorsal > pectoral > ventral area.
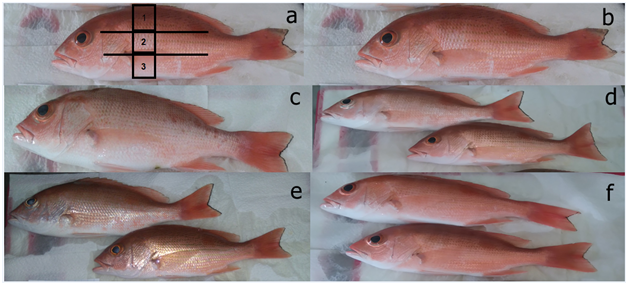
Figure 1 Color pattern to the naked eye of Pacific red snapper (a=sampling area, dorsal - I, pectoral - 2, ventral – 3); b=Wild; c=AX0; d=AX25; e=AX50; f=AX100).
|
Body parts /Parameters |
Lightness |
Redness |
Yellowness |
Chroma |
Hue |
Color |
|---|---|---|---|---|---|---|
|
L* |
a* |
b* |
Cab* |
H°ab |
||
|
Wild |
||||||
|
Dorsal |
39.35±0.02d |
33.07±0.07a,b |
13.92±0.12c |
35.88±0.01b |
22.68±0.73b |
Orange-red |
|
Pectoral |
42.39±0.03c |
29.98±0.23d |
12.96±0.15c |
32.66±0.26c |
23.24±0.0b |
Orange-red |
|
Ventral |
57.41±0.05b |
22.91±0.20b |
7.66±0.40d |
24.16±0.08d |
18.37±1.03c |
Red |
|
AX0 |
||||||
|
Dorsal |
40.84±0.29d |
35.97±0.24a |
22.25±0.27b |
42.83±0.11a,b |
32.67±0.28a |
Orange |
|
Pectoral |
43.88±0.14c |
30.00±0.20b |
18.57±0.23b |
35.28±0.19b,c |
31.55±0.19a |
Orange |
|
Ventral |
68.90±0.26a |
27.08±0.31c |
11.50±0.16c |
29.42±0.24c |
22.87±0.04b |
Orange-Red |
|
AX25 |
||||||
|
Dorsal |
41.06±0.02d |
36.19±0.42a |
23.37±0.42a,b |
42.55±0.58a,b |
31.52±0.18a |
Orange |
|
Pectoral |
44.10±0.03c |
30.22±0.54b |
18.25± 0.03b |
35.31±0.45b,c |
30.94±0.49a |
Orange |
|
Ventral |
69.12±0.05a |
27.30±0.23c |
10.02±0.35c,d |
29.08±0.33c |
20.03±0.50b,c |
Red |
|
AX50 |
||||||
|
Dorsal |
42.73±0.51c,d |
37.86±0.38a |
25.04±0.09a |
45.39±0.21a |
33.27±0.47a |
Orange |
|
Pectoral |
45.77±0.50c |
31.89±0.39b |
20.46±0.28b |
37.89±0.64b,c |
32.47±0.18a |
Orange |
|
Ventral |
70.79±0.42a |
28.97±0.28b,c |
13.39±0.16c |
31.91±0.28c |
24.66±0.11b |
Orange-red |
|
AX100 |
||||||
|
Dorsal |
44.52±0.31c |
39.65±0.21a |
27.50±0.11a |
48.25±0.21a |
34.52±0.29a |
Orange |
|
Pectoral |
47.56±0.24c |
33.68±0.41a,b |
22.25±0.27b |
40.36±0.24b |
33.23±0.32a |
Orange |
|
Ventral |
72.58±0.29a |
30.76±0.53b |
15.18±0.19c |
34.30±0.21c |
26.11±0.24b |
Orange-red |
Table 1 L*, a*, b*, *Cab and H°ab colour parameters from Pacific red snapper preference analyses
Superscripts in the same column with different letters are statistically different (P<0.05).
|
Treatments/Body parts |
Dorsal |
Pectoral |
Ventral |
|
Wild |
1.08±0.21e |
0.73±0.25e |
0.61±0.41e |
|
AX0 |
1.38±0.22d |
1.10±0.37d |
0.98±0.11d |
|
AX25 |
1.95±0.27c |
1.57±0.29c |
1.55±0.27c |
|
AX50 |
2.28±0.26b |
1.81±0.34b |
1.95±0.17b |
|
AX100 |
2.72±0.34a |
2.19±0.28a |
1.99±0.2a |
Table 2 Total carotenoids contents (mg/kg) in Pacific red snapper
Superscripts in the same column with different letters are statistically different (P<0.05).
None.
The author declares there is no any conflict of interest.

©2018 Carvajal-García, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.