Open Access Journal of
eISSN: 2575-9086


Research Article Volume 1 Issue 4
1Faculty of applied sciences and Humanities, Invertis University, India
2Department of Plant Science, MJP Rohilkhand University, India
Correspondence: Mohammad Mazid, Faculty of Applied Sciences and Humanities, Invertis University, India
Received: October 31, 2017 | Published: November 29, 2017
Citation: Mazid M, Naz F. Effect of macronutrients and gibberellic acid on photosynthetic machinery, nitrogen-fixation, cell metabolites and seed yield of chickpea (Cicer arietinum L.). Open Access J Sci. 2017;1(4):120-129. DOI: 10.15406/oajs.2017.01.00024
An experiment was carried out with an aim to enhance the performance of chickpea by the spray of a small quantity of phosphorus (P) and/or sulphur (S) with or without the soaking of GA treatment (10-6 M GA for 8h) and/ or the GA spray treatment (10-6 M GA at 60-70 DAS). P and S each at 2kg/ha were sprayed in two equal splits i.e. half at 60 and the remaining half at the 70 DAS alone or in combination with the GA treatment. Prior to sowing, total seeds of chickpea were grouped into two; one group of seeds was soaked in 0M GA (control) and the other group were soaked in 10-6 M GA aqueous solution, each for 8 hours. There were total sixteen treatments with ten combinations of P and/or S with GA are possible viz., FPS, SGA + FP, SGA + FS, SGA + FPS, FGAP, FGAS, FGAPS, SGA + FGAP, SGA + FGAS and SGA+FGAPS. The combined application of P and S with GA showed better responses and further improvement in carbonic anhydrase (CA) activity, stomatal conductance (gs), net photosynthetic rate (PN), nitrate reductase activity (NR), and leghemoglobin content (Lb) at two sampling stages (90 and 100 DAS). This treatment also increased pod number per plant, seed yield per plant and harvest index (HI), seed protein and carbohydrate content at harvest. This combination augmented the protein content (21%), carbohydrate content (11%), seed yield (86%) and HI (91.78%).
Keywords: gibberellins, carbonic anhydrase, nitrate reductase, photosynthesis, chickpea, leghemoglobin, yield
LB, leghemoglobin content; CA, carbonic anhydrase; PGRs, plant growth regulators
Pulses are well known as good rotational crops and have achieved important place in the Indian cropping system to buildup sustainable agriculture.1 Among pulses, for production, chickpea (Cicer arietinum L.), occupies the first position in India and third position at global level after bean and soybean.2,3 Chickpea belongs to the family Leguminosae and one important grain legume cultivated in the world and principle component of Golden Revolution as well. It has multiple functions in the traditional farming systems. It is very nutritive and used as a protein adjunct to starchy diets. It is given as preventive diet to atherosclerosis and diabetes patients because of its rich source of Ca, P, Mg, Fe, and K content.2 Its seeds contain about 20-29% protein, 59-61% carbohydrate, soluble sugars,3 8% 3-4% fat, 3% fiber and 3% ash.4,5 Moreover, its cultivation helps in sustaining soil fertility by fixing nitrogen (N) and meets 80% of its N requirement from symbiotic N fixation and can fix up to 140 kilogram per hectare (kg/ha) per year from air,6 particularly in the dry and rain fed area. In addition, it is also widely used as green manure. India is the largest producer of chickpea followed by Australia, Pakistan and Turkey.3
This crop is grown on 8.21million hectares of our country with the annual production of 7.48million tonnes and average productivity of 911kg/ha (FAO 2012). Though chickpea is grown in our country in the largest area in comparison with the other countries of the world, but her productivity at 911kg/ha is much lower than those of the developed countries of world, such as 2833.3kg/ha of China, 1668.4kg/ha of Canada and 1488.6kg/ha of USA (FAO 2012). The productivity of chickpea is often low due to heavy flower drop and pod shedding leading to poor seed setting which have been shown not as a result of insect damage, but due to physiological mechanism.7 Farmers have a wrong notion that chickpea being a legume crop, does not need any nutrition and usually grow it on the marginal lands, without applying any fertilizer. The yield gap of chickpea may be attributed to improper agro-technology used by the farmers. Yield gap can be abridged, by adopting the advanced production technology accompanying with the use of PGRs, balanced nutrition, weed management and selection of high yielding varieties.8 Low inorganic matter content in the soil is one of the major causes of the deficiency of nutrients.9 Due attention towards nutrient management is not paid in case of low input high risk rain fed legume crops, frequently grown in low fertility soils.10 To maintain the fertility of soils, the supplies must compensate what was exported at the harvest time. The reports are generally uniform and reliable simple to manage.
They are, drawn from non-renewable resources11 Plant growth regulators (PGRs) and macronutrients are known to play a positive role in regulating flower drop, premature pods development, and enhancing yield potential in plants. The rain fed crop records low biomass production due to inadequate soil moisture and nutrient availability ultimately resulting in less yields. Among the multi-nutrient deficiencies, P and S deficiency is posing a serious problem particularly in pulse crops owing to their higher requirement of these nutrients. Diammonium phosphate is most commonly used in pulse crops which supplies N and P but not S. Moreover, limited use of single superphosphate aggravated S efficiency in soil. Thus, the decline in yield of chickpea has been mainly attributed to the deficiencies of P and S which are common in most of the soil.12 The production of chickpea has not keeping pace with the increasing domestic demands. PGRs help to increase the number of flowers, regulation of flowering and their retention,13 since the flowering is influenced by PGRs, the number of pods increases which results in an increase in yield.14,15 The PGRs also influences various growth and biochemical parameters. Gibberellins are considered to be the most florigenic of known PGRs.
To meet the challenges of the low chickpea production and local requirements, there is need for multipronged strategy. In this context, efforts in the form of launching national programs and research projects have been made by governmental and non-governmental organizations. However, it must be admitted that these efforts howsoever laudable have not yet succeeded in offsetting the undesired shortfall in the indigenous chickpea-market. Evolving as well as adopting the best strategy for triple-purpose crop that may be one of the endeavors to improve the situation of chickpea shortage. This would be possible through the increase the height of the plant and to improve seed yield. To attain such goal, the use of GA3 and P and S may play an important role as they are known to affect many facets of plant life including photosynthetic rate (PN), N-fixation, water and mineral uptake, harvest index, in regulation of growth and development by enhancing cell elongation and cell differentiation thus augmenting plant height and seed yield.16
Moreover, GA3 inhibit the adventitious root formation but enhance a number of physiological processes including activity of ribulose-1,5-bisphosphate carboxylase (RuBPcase), bunching of grapes, breaking of seed and bud dormancy, cell-wall plasticity, cell elongation, flowering, growth and yield of sugarcane, PN, parthenocarpy, protein-synthesis, phloem-loading, relative growth rate (RGR), stomatal aperture, senescence, stem-elongation, seed-germination, synthesis and secretion of hydrolyzing enzymes particularly α-amylase for promoting hydrolysis of storage-reserves, transpiration rate, transcription of messenger (m)-RNA and vernalization and thus augmenting seed yield.17‒20 However, plants cannot respond maximally to GA3 when the supply of nutrients is inadequate (Khan et al. 2002a). Moreover, the requirement of chickpea for nutrients is very high and further the intensive cropping systems may remove much of the applied nutrients under high productivity conditions.21
A considerable amount of fertilizers is rendered unavailable to the plant as it grows due to many factors, including leaching, fixation, decomposition and volatilization. For example, up to 50% of the soil-applied N,22 about 70% of the soil-applied P23 and as reported by,24 more or less 60% of the total applied S may be lost due to one or more of these reasons. Among mineral nutrients, P is an essential nutrient and an integral component of several important compounds, including adenosine triphosphate and other related high energy compounds, all sugar-phosphates in photosynthesis, co-enzymes, glycerol phosphatides, nucleic acids, nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, phospholipids and phosphoglycerides.25 S is also represents the ninth and least abundant essential macro-elements in plants, proceeded by C, O, H, N, K, Ca, Mg and P. S plays critical roles in the catalytic and/or electro-chemical functions of the biomolecules in cells and also as signaling molecules for fundamental cellular functions.26 S is a constituent of many organic compounds, including alliins, biotin, co-enzyme-A, cysteine and methionine, ferredoxins, lipoic acid, glucosinolates, thiamine and thiamine-pyrophosphate.27
Ferredoxins are also important for nitrogen assimilation. Thus, S plays an important role in plant growth and in the regulation of plant development and required for protein, lipids, carbohydrates synthesis, NR and electron transport systems functionality. Considering the overwhelming importance of Cicer arietinum L. in soil fertility recovery as well as determination of the growth, development and yield responses of chickpea to GA3, P and S application singly or in combination, a experiment was laid out to test whether the spray of P and /or S in the presence or absence of GA3 will improve the performance of chickpea.
Experimental site
Aligarh is one of the seventy five districts of Uttar Pradesh.28 It is situated at 27.88˚N latitude,78.08°E longitude and 180 m average altitude with an area of 3700.4 sq km. Its climate is sub-tropical, with severest hot dry summers and intense cold winters. The winter extends from the middle of October to the end of March. The average temperatures for December and January are about 15°C and 13°C respectively. The recorded extremely lower temperatures for any single day are 2°C and 0.5°C respectively. The summer extends from April to the end of June. The average temperatures are 34.5°C and 34°C while, the extreme maximum records of temperatures are 45°C and 45.5°C for May and June respectively. The monsoon extends from the end of June to the middle of October and the mean temperature ranges between 26°C to 30°C. The average rainfall is 847.3mm. More than 85% of the total rainfall occurs during a short span of four months from June to September and the remaining showers are received during winter season, useful for Rabi crops. Some additional occasional rainfall during the summer is very rare, sporadic, and variable to a greater extent in amounts. 4% out of total rainfall occurs during this season on an average scale. The relative humidity of the winter season ranges between 56% to 77% with an average 66.5% that of the summer between 37% and 49% with an average of 43% and that of the monsoon seasons, between 63% and 73% with an average of 68%. Aligarh district has the same soil composition and the appearances as those found generally in the plains of western Uttar Pradesh (Northern India). Different types of soil, such as sandy, loamy, sandy-loam and clayey loam are found in the district.
Plant materials
Authentic seeds of a newly released high yielding cultivar of chickpea, namely DCP 92-3 was obtained from the IIPR, Kanpur (Uttar Pradesh). After selecting seeds of uniform size, their viability was tested. The healthy seeds were soaked with double distilled water (DDW) for 2h and then were surface sterilized with absolute ethyl alcohol followed by repeated washing with DDW. Subsequently, seeds were inoculated with the recommended strain of Rhizobium namely TAL 1148 and then were sown in earthen pots. The crop was shown on the 20th October, 2012 and harvested on the 25th March, 2013.
Soil analysis and experimental design
Just before sowing a composite soil sample, collecting randomly from each pot containing the homogenous mixture of soil and FYM (4:1) was analysed for the soil characteristics. The soil sample was analysed in the Soil Testing Laboratory, Government Agriculture Farm, Quarsi, and Aligarh for various physico-chemical properties. Texture, sandy loam; pH, 8.04; E.C.(dSm-1), 0.65; N-P-K(Kg/ha), 210-34.32-209.20 respectively and calcium carbonate (%),0.10. Before sowing, the earthen pots of equal size (25cm heightx25cm diameter) were filled with the homogenous mixture of soil and FYM in the ratio of 4:1 at the rate of 5kg /pot. The required number of pots was arranged according to a simple randomized design in a net house of the Department of Botany. Just one day before the sowing, pots were irrigated lightly to provide necessary moisture for seed germination.
Preparation of solutions of PGR and nutrients
Prior to the foliar treatments, 100 milliliter (ml) stock solution of GA at 10-3M was prepared. The amount of GA was dissolved in 10ml ethyl alcohol and the final volume was made 100ml using DDW. Further dilutions of the stock solutions were made with DDW as per requirement. P and S solution at 0.1% was used for foliar spray. For application of nutrients alone, DDW was used as solvent however, the hormone solution was treated as solvent when nutrients and hormone were applied together. The aim of this experiment was to maximize the performance of cultivar DCP 92-3 by the spray of a small quantity of P and/or S with or without the best soaking GA treatment (10-6 M GA for 8h), and/or the GA spray treatment (10-6 MGA at 60-70 DAS) determined. P and S each at 2kg/ha were sprayed in two equal splits, i.e. half at 60 and the remaining half at the 70 DAS alone or in combination with the GA treatment. The sources of P and S were sodium dihydrogen-orthophosphate and sodium sulphate respectively. There were in all sixteen treatments with four replicates.
Sampling and data collection
One plant from each replicate was uprooted randomly at the various sampling stages to assess the performance of the crop on the basis of growth characters, physiological and biochemical characteristics, yield attributes and quality parameters. Physiological and biochemical characteristics were studied at 90 and 100 DAS while yield and quality parameters at harvest. The chlorophyll content was estimated in fresh leaves collected randomly from each replicate by the method of Arnon (1949). PN is the total rate of photosynthetic CO2 fixation minus the rate of loss of CO2 during the respiration. The PN was measured in fully expanded leaves of somewhat the same age in all replicates by using the Infra Red Gas Analizer (IRGA), LICOR-6400, Nebraska, and USA). Each observation was repeated thrice. All the measurements were made on cloudless clear days between 11.00 O’ clock and 12.00 Noon. The PN was expressed in terms of µ mol (CO2) m-2 second (s)-1. gs is a numerical measure of the maximum rate of passage of either water vapour or CO2 through the stomata.
It was also measured by the IRGA simultaneously with PN. Gs were expressed in terms of mol m-2 s-1. CA activity was determined in fresh leaves collected randomly from each replicate. The enzyme CA catalyzes the reversible hydration of CO2 to give the bicarbonate ion (HCO3-). The activity of the enzyme was estimated by adopting the method of.29 The NR activity in fresh leaves was estimated by the method of.30 The leghaemoglobin content in fresh nodules was estimated following the method described by Sadasivam and.31 To assess the yield performance of the crop, the remaining two plants from each pot were harvested at maturity. The harvested plants were sun-dried in a net-house to prevent losses. After drying the crop, each sample was threshed individually. Number of pods per plant was determined at physiological maturity from two remaining plants. The pods were manually removed from all the harvested plants and number of pods per plant determined. The seeds were utilized for assessing the other characteristics. The total seeds of two plants were threshed, cleaned and allowed to dry in the sun for some time and their weight was obtained with the help of an electronic balance, with expressing their weight on per plant basis. The proportions of the biological yield representing the economic yield are called HI. The HI was computed by dividing the seed yield (economic yield) of a plant by the biological yield of the plant and expressed on percent basis. HI was calculated by the following formula:
The total seed protein and carbohydrate content in the dry seeds was estimated by adopting the methodology of.32 All data were analyzed statistically adopting the analysis of variance technique, according to.33 In applying the F test, the error due to replicates was also determined. When ‘F’ value was found to be significant at 5% level of probability, critical difference (CD) was calculated.
Photosynthetic variables
Treatment SGA+ FGAPS gave the maximum value of chlorophyll content at both sampling stages. Its effect was, however, equal to that of SGA+ FGAP, SGA+ FGAS, SGA+ FGA, FGAS and FGAP at each stage of sampling and also to that of FGA at 90 DAS. Treatment SGA+ FGAPS gave 46.16 and 48.84% higher value at 90 and 100 DAS respectively than FW (Table 1). At 90 DAS, treatment SGA+FGAPS gave the maximum value of PN and its effect was however, at par with that of SGA+FGAS and SGA+FGAP. At 100 DAS, treatment SGA+FGAS gave the highest value, with its effect being by that of SGA+FGAP and SGA+FGAPS (Table 2). Treatment SGA+FGAPS gave 64.24 and 65.37% higher value at 90 and 100 DAS respectively than FW. The effect of treatments on gs was found non-significant at both stages of sampling. The effect of treatments on gs was found non-significant at both stages of sampling (Table 3). Treatment SGA+ FGAPS gave the maximum value of CA at 90 DAS. Its effect was, however, equal to that of SGA+ FGAS, SGAPS, FGA+ FGAP and FGAPS at 90 DAS. Treatment SGA+ FGAPS gave 64.56% higher value than FW at 90 DAS. However, this parameter was not affected by treatments at 100 DAS (Table 4). Treatment SGA+FGAPS gave the maximum value of NR at each sampling stage. Its effect was, however, equaled by that of SGA+ FPS, FGAPS, SGA+FGAS at both stages and was also by that of SGA+FS at 100 DAS. Treatment SGA+FGAPS gave 22.37 and 22.46 % higher value at 90 and 100 DAS respectively than FW (Table 5). Treatments SGA+FGAPS and SGA+FGAS were at par in their effect and gave maximum value of Lb at each stage of sampling. Treatments, FGAPS, SGA+FGAP and FGAS being at par occupied second position at each stage and treatments FGAP, SGA+FPS and FGAS also at 90 DAS. Treatment SGA+FGAPS gave 206.13 and 215.38% higher value at 90 and 100 DAS respectively than FW (Table 6).
|
Chlorophyll Content [Mg G-1 (F.M.) ] |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
1.69 |
1.72 |
|
FP |
1.81 |
1.84 |
|
FS |
1.94 |
1.97 |
|
FPS |
1.97 |
2 |
|
SGA |
2.14 |
2.21 |
|
SGA+FP |
2.17 |
2.2 |
|
SGA+FS |
2.19 |
2.2 |
|
SGA+FPS |
2.24 |
2.29 |
|
FGA |
2.37 |
2.39 |
|
FGAP |
2.39 |
2.4 |
|
FGAS |
2.35 |
2.38 |
|
FGAPS |
2.4 |
2.41 |
|
SGA+FGA |
2.42 |
2.5 |
|
SGA+FGAP |
2.44 |
2.53 |
|
SGA+FGAS |
2.43 |
2.49 |
|
SGA+FGAPS |
2.47 |
2.56 |
|
C.D. at 5% |
0.156 |
0.56 |
Table 1 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on chlorophyll content of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
|
Net Photosynthetic Rate (M M CO2 M-2 S-1) |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
7.16 |
6.64 |
|
FP |
8.1 |
7.54 |
|
FS |
8.27 |
7.84 |
|
FPS |
9.11 |
8.84 |
|
SGA |
7.61 |
8.2 |
|
SGA+FP |
8.64 |
8.29 |
|
SGA+FS |
8.94 |
8.4 |
|
SGA+FPS |
9.27 |
8.24 |
|
FGA |
9 |
8.97 |
|
FGAP |
10.44 |
9.92 |
|
FGAS |
10.45 |
9.97 |
|
FGAPS |
10.4 |
10.13 |
|
SGA+FGA |
10.4 |
10.1 |
|
SGA+FGAP |
11.44 |
10.87 |
|
SGA+FGAS |
11.54 |
10.98 |
|
SGA+FGAPS |
11.76 |
10.7 |
|
C.D. at 5% |
0.68 |
0.64 |
Table 2 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on net photosynthetic rate of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
|
Stomatal Conductance (M Mol M-2 S-1) |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
0.19 |
0.17 |
|
FP |
0.192 |
0.17 |
|
FS |
0.197 |
0.172 |
|
FPS |
0.197 |
0.177 |
|
SGA |
0.2 |
0.192 |
|
SGA+FP |
0.211 |
0.193 |
|
SGA+FS |
0.213 |
0.19 |
|
SGA+FPS |
0.213 |
0.2 |
|
FGA |
0.218 |
0.201 |
|
FGAP |
0.223 |
0.207 |
|
FGAS |
0.224 |
0.21 |
|
FGAPS |
0.218 |
0.21 |
|
SGA+FGA |
0.211 |
0.211 |
|
SGA+FGAP |
0.226 |
0.211 |
|
SGA+FGAS |
0.217 |
0.209 |
|
SGA+FGAPS |
0.227 |
0.225 |
|
C.D. at 5% |
NS |
NS |
Table 3 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on stomatal conductance of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
|
Carbonic Anhydrase Activity [Mol CO2 Kg-1 (F.M.) S-1 ] |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
2.37 |
2.49 |
|
FP |
2.39 |
2.81 |
|
FS |
2.46 |
2.74 |
|
FPS |
2.74 |
2.87 |
|
SGA |
3.41 |
3.42 |
|
SGA+FP |
3.44 |
3.58 |
|
SGA+FS |
3.5 |
3.63 |
|
SGA+FPS |
3.78 |
3.92 |
|
FGA |
3.57 |
3.6 |
|
FGAP |
3.63 |
3.65 |
|
FGAS |
3.74 |
3.77 |
|
FGAPS |
3.84 |
3.9 |
|
SGA+FGA |
3.71 |
3.76 |
|
SGA+FGAP |
3.79 |
3.8 |
|
SGA+FGAS |
3.85 |
3.86 |
|
SGA+FGAPS |
3.9 |
3.94 |
|
C.D. at 5% |
0.236 |
NS |
Table 4 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on carbonic anhydrase activity [mol CO2 kg-1(F.M.) s-1 ] of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
|
Nitrate Reductase Activity N [Mol NO2- / G/ (Leaf F W)/ H] |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
300 |
305.42 |
|
FP |
320.4 |
327.1 |
|
FS |
337.8 |
342.45 |
|
FPS |
340.1 |
347.1 |
|
SGA |
302.4 |
309.2 |
|
SGA+FP |
309.4 |
313.4 |
|
SGA+FS |
341.3 |
350.2 |
|
SGA+FPS |
357.2 |
363.4 |
|
FGA |
304.1 |
307.2 |
|
FGAP |
307 |
319.4 |
|
FGAS |
317.3 |
330.2 |
|
FGAPS |
353.4 |
357.9 |
|
SGA+FGA |
327.1 |
341.2 |
|
SGA+FGAP |
340.2 |
347.4 |
|
SGA+FGAS |
347.2 |
370.4 |
|
SGA+FGAPS |
367.1 |
374 |
|
C.D. at 5% |
23.44 |
24.01 |
Table 5 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on nitrate reductase activity of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
|
Leghaemoglobin Content Mg G-1 (F.M)) |
||
|---|---|---|
|
Treatments |
90 DAS |
100 DAS |
|
FW |
0.147 |
0.13 |
|
FP |
0.157 |
0.15 |
|
FS |
0.18 |
0.16 |
|
FPS |
0.195 |
0.18 |
|
SGA |
0.34 |
0.31 |
|
SGA+FP |
0.36 |
0.33 |
|
SGA+FS |
0.35 |
0.305 |
|
SGA+FPS |
0.36 |
0.3 |
|
FGA |
0.35 |
0.31 |
|
FGAP |
0.39 |
0.33 |
|
FGAS |
0.37 |
0.37 |
|
FGAPS |
0.39 |
0.365 |
|
SGA+FGA |
0.38 |
0.31 |
|
SGA+FGAP |
0.4 |
0.35 |
|
SGA+FGAS |
0.43 |
0.41 |
|
SGA+FGAPS |
0.45 |
0.4 |
|
C.D. at 5% |
0.024 |
0.026 |
Table 6 Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on Leghaemoglobin content of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
N.B: A uniform basal dose of 40 kgN+30kg P2O5/ha was given to all pots.
PGRs are important control agents for growth and development of plants.34‒38 In view of their crucial roles in different facets of plant life and very small quantity involved (economic), it is reasonable to use of PGRs in innovative farm cultural practices. Among PGRs, GA occupies a prominent position in mediating a variety of plant physiological processes including seed germination, leaf expansion, flower and fruit set, dry matter production, photosynthesis, translocation of food material and synthesis of mRNA coding for hydrolytic enzymes.39‒45 The superiority of GA to the above mentioned PGRs has also been substantiated in the author’s preliminary experiment.46 Keeping its prominent role in various physiological processes of plants, it is logical to exploit its potential by way of establishing its (i) adequate level and soaking duration for pre-sowing seed treatment, appropriate concentration and operational growth stage/s for foliar application and, mode of application (through foliage and /or seeds).
The vegetative and reproductive growth of plants depends mainly on their ability to fix C in organs having chloroplasts followed by the utilization of the photosynthates for sink organs. As the C fixing ability of plants is influenced by mineral elements among other factors, the availability of P and S to leguminous plants affects production of dry matter and partitioning of photosynthates.47 The enhancing effect of foliar application of 10-6 MGA at 60-70 DAS over the water-sprayed control on CA, NR, PN, gs, and Lb (observed at 90 and 100 DAS) of chickpea cultivars, particularly DCP 92-3 receiving the officially recommended basal dose of 40kgN+30kg P2O5/ ha can be traced to its various comparatively more roles in plants. For example, application of GA improves, among other processes, absorption and use efficiency of nutrients,48,49 activity of enzymes,50,51 cell division and cell enlargement,52,53 chlorophyll content,54 elongation of internode, membrane permeability,55‒57 PN,58 nucleic acid and protein synthesis,59‒61 and transport of photosynthates.62 The improving effect of the spray of a small quantity of P and /or S alone or in combination with the soaking and/or foliar spray treatment of GA over the respective control on the growth parameters (observed at 90and100DAS) of chickpea cultivar DCP 92-3 grown with the recommended basal dose of N and P is a noteworthy observation.
The promoting effect of P and S on the biochemical parameters can be traced to their previously mentioned various roles in introduction. S also helps in chlorophyll formation63,64 and stimulates root growth.65 Thus, being important essential nutrients, P and S are directly or indirectly involved in growth of chickpea like other crops through the production of metabolic compounds. These metabolites, in turn, encourage the formation and enlargement of new cells in treated plants. It may be added that these results on the improving effect of foliar application of P and S broadly corroborate the earlier findings of.66,67 The augmenting effect of leaf-applied GA over the water-sprayed control on CA and NR activities of chickpea cultivars particularly DCP 92-3, receiving the recommended basal dose of 40kg N+ 30kg P2O5/ha, studied at 90 and 100 DAS is worth mentioning. The increase in CA and NR activities can be attributes to the hormone-induced increase in transcription and/or translation of the gene that codes for CA68,69 and NR70,71 to its role in enhancing the permeability of membranes and absorption of nutrients. These results are also in accordance with the data of earlier workers including72,73 and14 on CA activity;74‒76 on NR activity; and77‒80 on NPK content.
The enhancing effect of pre-sowing seed treatment for 8 h and foliar treatment at 60 and 70 DAS with 10-6 MGA over their respective water treated control on CA and NR activities of DCP 92-3 cultivar of chickpea grown with the recommended basal dose of N and P is a noteworthy observation. This may also be attributed, as for growth characters, to its (GA) roles on one hand and compensation of the ‘hidden hunger’ for GA by its pre-sowing seed treatment or foliar application on the other. These results also corroborate the findings of81,82 on CA activity, of81,82 on NR activity and of82 for pre-sowing seed treatment and of those mentioned on earlier for these parameters for foliar application of GA. The augmenting effect of foliar spray of P and /or S alone or in combination with the soaking and /or foliar spray treatment of GA over the respective control on CA activity, NR activity of chickpea cultivar DCP 92-3 grown with the recommended basal dose of N and P is not far to seek. P and S being component of the various metabolites involve in the production of organic compounds which in turn encourage the formation and proper supply of proteins to be involved in the formation of enzymes. Hence higher activity of CA and NR in treated plants.
Enhanced rate of CA activity of chickpea cultivar DCP-92-3 would have resulted in improving the PN and gs of treated plants (for PN and for gs). Likewise, increased NR activity might be responsible for increasing biosynthesis of chlorophylls that in turn would have improved PN of treated plants. Improvement in N, P and K content of cultivar DCP 92-3 (data not published) would also have enhanced content of Lb content on the other. Higher levels of Lb content would also be responsible for increased content of chlorophylls leading to higher PN. This proposition is further confirmed by correlation studies emphasizing a positive and significant correlation between these pairs of parameters.
Yield attributes and quality parameters
Treatment SGA+FGAPS gave the maximum value for pod number per plant. Its effect was, however, equal to that of SGA+FGAS. Treatment SGA+FGAPS gave 121.72% higher value than FW (Table 7). Treatment SGA+FGAPS gave the maximum value of seed yield. Its effect was, however, equal to that of SGA+FGAS, SGA+FGAP and SGA+FGA. Treatment SGA+FGAPS gave 86.14% higher value than FW (Figure 1). Treatment SGA+FGAPS proved best for HI. Its effect was, however, equal to that of SGA+FGAS, FGAPS, SGA+FGAP, SGA+FPS and FGAP. Treatment SGA+FGAPS gave 91.78% higher value than FW (Table 7). Treatment SGA+FGAPS gave the maximum value for quality parameters viz., seed protein and carbohydrate content. Its effect was, however, equal to that of FGAPS, FGA, SGA+FGA, SGA+FGAS, FGAS, SGA+FGAP, SGA+FS, SGA+FP, FGAP, SGA and FPS. Treatment SGA+FGAPS gave 20.59% and 11% higher value than FW respectively (Figure 2 & Table 8). The increase in the number of pods per plant and 100-seed weight resulting from the foliar application of GA in comparison with the water-sprayed control of chickpea cultivar DCP 92-3 receiving the recommended basal dose of N and P in is worth mentioning. The increase in the above yield attributes may be traced to its various roles mentioned earlier leading to observed higher values for growth characters and, physiological and biochemical parameters of treated plants.
|
Treatments |
Pod Number Per Plant |
|
FW |
15.2 |
|
FP |
17.4 |
|
FS |
18.25 |
|
FPS |
20.7 |
|
SGA |
24.6 |
|
SGA + FP |
25.1 |
|
SGA + Fs |
28.9 |
|
SGA+ FPS |
27.4 |
|
FGA |
27.4 |
|
FGAP |
29.5 |
|
FGAS |
30.2 |
|
FGAPS |
30.7 |
|
SGA + FGA |
28.9 |
|
SGA+FGAP |
29.1 |
|
SGA + FGAS |
33.4 |
|
SGA+FGAPS |
33.7 |
|
C.D. at 5% |
1.85 |
Table 7: Effect of spray of P and/ or S in the presence or absence of soaking and / or spray treatment of GA on pod number per plant of chickpea cultivar DCP 92-3 at harvest (mean of four replicates).
N.B: A uniform basal dose of 40kg N+ 30 kg P2O5 / ha was given to all pots.
|
Treatments |
Harvest index (%) |
|---|---|
|
FW |
30.4 |
|
FP |
41.2 |
|
FS |
39.4 |
|
FPS |
43.7 |
|
SGA |
49.45 |
|
SGA + FP |
50.2 |
|
SGA + Fs |
53.4 |
|
SGA+FPS |
55.1 |
|
FGA |
52.2 |
|
FGAP |
54.75 |
|
FGAS |
53.1 |
|
FGAPS |
55.9 |
|
SGA +FGA |
53.8 |
|
SGA+FGAP |
55.2 |
|
SGA + FGAS |
57.85 |
|
SGA+FGAPS |
58.3 |
|
C.D. at 5% |
NS |
Table 8 Effect of spray of P and/ or S in the presence or absence of soaking and / or spray treatment of GA on harvest index of chickpea cultivar DCP 92-3 at harvest (mean of four replicates).
N.B: A uniform basal dose of 40 kg N + 30 kg P2O5 / ha was given to all pots.
|
Treatments |
Seed carbohydrate content (%) |
|
FW |
65.2 |
|
FP |
66.15 |
|
FS |
69.7 |
|
FPS |
70 |
|
SGA |
70.2 |
|
SGA + FP |
71 |
|
SGA + Fs |
70.4 |
|
SGA+ FPS |
69.8 |
|
FGA |
66.7 |
|
FGAP |
67.8 |
|
FGAS |
69.1 |
|
FGAPS |
70.8 |
|
SGA +FGA |
68.4 |
|
SGA+FGAP |
70.9 |
|
SGA + FGAS |
71.6 |
|
SGA+FGAPS |
72.4 |
|
C.D. at 5% |
2.04 |
N.B: A uniform basal dose of 40 kg N + 30 kg P2O5/ha was given to all pots.
Table 9 Effect of spray of P and/ or S in the presence or absence of soaking and / or spray treatment of GA on seed carbohydrate content of chickpea cultivar DCP 92-3 at harvest (mean of four replicates).
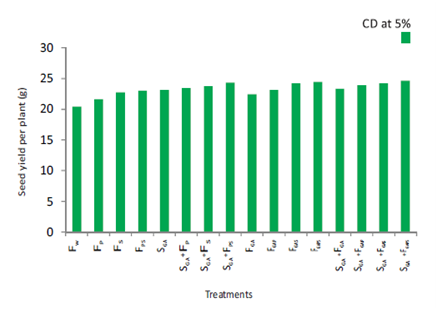
Figure 1 Effect of spray of P and / or S in the presence or absence of soaking and / or spray treatment of GA
on seed yield per plant of cultivar DCP 92-3 of chickpea.
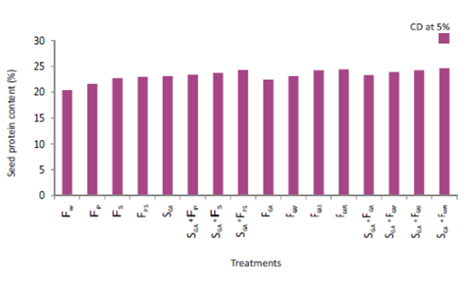
Figure 2 Effect of spray of P and / or S in the presence or absence of soaking and / or spray treatment of GA on seed protein content of cultivar DCP 92-3 of chickpea.
Moreover, it mediates differentiation leading to enhanced number of flowers which develop into pods. As mentioned earlier, it plays role in cell division and cell enlargement 25‒30 resulting in proper development of under-developed pods especially at the terminal end of branches; PN31,32 supplying sufficient C skeleton; and membrane permeability and transport of photosynthates83‒85 favoring partitioning hence higher values for the yield parameters of treated plants. These results broadly corroborate the findings of,86 and Shah & Samiullah. The augmenting effect of pre-sowing seed treatment with 10-6 MGA for 8h over water-soaking treatment on pods per plant and seeds per pods and of spray treatment at 60 and 70 DAS with the same concentration of GA in comparison with the water-sprayed control on these parameters as also on 100-seed weight in conducted both on chickpea cultivar DCP 92-3 grown with a recommended basal dose of N and P, is understandable. This may be due to its roles mentioned on earlier for improving these parameters and offset of the ‘hidden hunger’ for GA by its pre-sowing seed treatment or foliar application. Similar results were also obtained by65−67 on pre-sowing seed treatment and by those mentioned on foliar application of GA.
The improvement in pods per plant, seeds per pod and 100-seed weight of chickpea cultivar DCP 92-3 grown with the recommended basal dose of N and P, due to foliar application of a small quantity of P and /or S alone or in combination with the soaking and /or spray of GA over the respective control is not far to seek. The increase in these parameters due to spray P and S can be ascribed to their roles mentioned earlier. Also, P and S influence differentiation in plants.86,87 The improvement in growth, physiological and biochemical parameters resulted from the spray of these nutrients together with enhancement in differentiation may lead to the improvement in yield parameters, hence higher values for pods per plant, seeds per pod and 100-seed weight. These results are in accordance with the findings of43−86 on foliar application of P and S. The increased yield attributing parameters of treated plants, particularly pods per plant and88,90 100-seed weight are likely to have contributed to the improved seed yield. This proposition is confirmed by correlation studies also wherein various yield characters may be noted to the positively and significantly correlated with seed yield.91−95 The observed increase in seed protein content due to foliar application and due to pre-sowing seed treatment of GA is not surprising. An improvement in protein synthesis may result from the application of GA and P and S, hence higher values for seed protein content.96−100 These results broadly corroborate with the findings of87 on GA application and of on P and S although on basal application. It is highly satisfying to note that the results of this pot experiment undertaken above clear show that the author’s hypothesis forming the basis of the endeavour under discussion stand confirmed.101−111
In the end, the present author wishes to claim that he has been able to enrich the scientific literature on chickpea by contributing the following new findings: The efficacy of a small quantity of leaf-applied P and S (each at 2kg/ha) along with the best soaking (10-6 M GA for 8h) and spray (10-6 MGA at 60 & 70 DAS) treatments was tested and the above combination of nutrients and GA treatments gave the best performance of chickpea cultivar DCP 92-3.
We are grateful to the Dean, R. K. Shukla, Faculty of Applied Sciences and Humanities, Bareilly, for providing research facilities. We are also grateful to Prof D.K. Saxena for his critical comments and valuable suggestions with regard to the preparation of the manuscript.
The author declares that there is no conflict of interest.

©2017 Mazid, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.