Open Access Journal of
eISSN: 2575-9086


Research Article Volume 1 Issue 4
1Department of Analytical and Organic Chemistry, Azerbaijan State Pedagogical University, Azerbaijan
2Department of Organic Chemistry, Baku State University, Azerbaijan
Correspondence: Ali Z Zalov, Department of Analytical and Organic Chemistry, Azerbaijan State Pedagogical University, Azerbaijan
Received: August 31, 2017 | Published: October 16, 2017
Citation: Zalov AZ, Maharramov AM, Huseynova AT, et al. Extraction and spectrophotometric determination of copper (ii) with 1-(2-metoxiphenylamin)-3- metoksipropanthiol-2. Open Access J Sci. 2017;1(4):97-102. DOI: 10.15406/oajs.2017.01.00019
1-(2-Metoxiphenylamin)-3-metoksipropanthiol-2 (MPAMPT) is proposed as an analytical reagent for the extractive spectrophotometric determination of copper (II). MPAMPT forms blue colored complex with copper (II) in the pH range 5.4-6.8. Beer’s law is obeyed in the concentration range up to 16μg mL−1. The yellowish Cu (II)-MPAMPT complex shows a maximum absorbance at 605nm, with molar absorptivity of 4.32×104dm3mol−1cm−1 and Sandell’s sensitivity of the complex obtained from Beer’s data is 1.48ngcm−2. The composition of the Cu (II)–MPAMPT complex is found to be 1:2 (M/L). The interference of various cations and anions in the method were studied. Thus the method can be employed for the determination of trace amount of copper (II) in pharmaceutical, food and plant sample.
Keywords: copper, 1-(2-metoxiphenylamin)-3-metoksipropanthiol-2, determination, chromomeric reagent, complex, automobile radiators, biologically active metal
The alloys of copper find extensive use in automobile radiators, heat exchangers, home heating systems and panels for absorbing solar energy. Copper (II) is a biologically active metal; its compounds affect the vital activity of plant and animal organisms. Copper is one of essential elements required for normal human metabolism.1 Copper (II) is known to play a significant role in biological systems and also as a pharmaceutical agent.2 Its antibacterial properties have been known for thousands of years. Synthetic copper(II) complexes have been reported to act as a potential anticancer and cancer inhibiting agents and a number of copper complexes have been found to be active both in vitro and in vivo.3,4 Therefore it is necessary to control that the content of copper (II) ions in the environmental objects is within the permissible concentrations.
Hence, it is necessary to seek highly, accurate and selective analytical methods for quantitative determination of copper at trace levels. Various spectrophotometric methods have been proposed for the determination of copper contents of the various samples including natural waters and pharmaceutical samples.5 For the spectrophotometric determination of copper in various sites(in water, alloys and pharmaceutical samples, synthetic mixtures) suggested naphthazarin (5,8-dihydroxy-1,4-naphthoquinone),6 1-phenyl-1-hydrazonyl-2-oximino propane -1, 2- dion,7 4-[N, N-(dimethyl) amino] benzaldehyde thiosemicarbazon,8 2-(5-bromo-2-oxoindolin-3-ylidene) hydrazine carbothioamide as an analytical reagent,9 4-(4’-nitrobenzylidene imino)-3-methyl-5-mercapto-1, 2, 4-triazole,10 2-hydroxy-3-methoxy benzaldehyde thiosemicarbazon.11 2-acetylthiophene thiosemicarbazone,12 o-hydroxyacetophenone isonicotinoylhydrazone.13 Oxyphenolate and dithiophenolate complexes of copper are insoluble in chloroform, while mixed-ligand complexes with hydrophobic amines and aminophenols easily dissolve in various organic solvents.14‒16 Copper (II) which forms an intense blue coloured complex with 1-(2-Metoxiphenylamin)-3-metoksipropanthiol-2 (MPAMPT), which is completely extracted into chloroform. This forms the basis of the proposed extractive spectrophotometric method for determination of copper after its extraction in the form of Cu(II)- MPAMPT complex. The method has been successfully applied to the analysis of a large variety of samples with diverse matrices such as pharmaceutical, food and plant samples.
Reagents and apparatus
The stock solution (1mg / ml) of Copper (II) was prepared by dissolving weighed amount of Copper Sulphate (CuSO4) in doubly distilled deionized water.17 More dilute standard solutions were prepared from this stock solution as and when required. Solutions of MPAMPT in chloroform (0.01M) were used. To create the optimal acidity, 0.1M solutions of KOH and HCl or ammonium acetate buffers were applied. Acetate buffer solution, prepared by mixing of 2mol x L−1 aqueous solutions of CH3COOH and NH4OH. The stock solution of various metal ions and anions were prepared by dissolving the appropriate metal salts in distilled water or with suitable dilute acids and making up to a known volume. The extractant was purified chloroform. The absorbance of the extracts was measured using a Shimadzu UV1240 spectrophotometer and KFK 2 photocolorimeter (USSR). Glass cells with optical path of 5 or 10mm were used. pH of aqueous phase was measured using an I-120.2 potentiometer with a glass electrode. Muffle furnace was used for dissolution of the samples. The process of thermolysis of the compounds was studied using derivatograph system «ShimadzuTGA-50H». IR spectra were recorded on a spectrophotometer "Specord M80" (Germany).
General procedure
General procedure for the determination of copper: Portions of stock solutions of Copper (II) varying from 0.1 to 1.0mL with a 0.1-mL step, a 2.0mL portion of a 0.01M solution of MPAMPT, and a 2.5 mL portion of a 0.01M solution of Am were placed in to calibrated test tubes with ground-glass stoppers (the volume of the organic phase was 5mL). The required value of pH was adjusted by adding 0.1M NaOH. The volume of the aqueous phase was increased to 20mL using distilled water. In 10minnute after the complete separation of the phases, the organic phase was separated from the aqueous phase and the absorbance of the extracts was measured on KFK-2 at room temperature and 590nm (l=0.5cm).
Procedure for determination of cu (ii) in pharmaceuticals samples: Boiling with 10ml of aqua regia dissolved 0.5gm of Pharmaceuticals samples. The solution was evaporated to dryness and the residue was dissolved in 10ml of 1M HCl filter, if required and resulting solution was diluted to 100ml with doubly distilled water. The working solution was prepared by appropriate dilution of stock solution. From an aliquot of this solution 1 ml was analysed for Cu (II) by the procedure as described earlier.
Determination of copper in plant: A portion of beans (10g) was crushed and dried in a porcelain dish at 105°С. The dry residue is heated in a muffle furnace at 500°С. The ash was dissolved in diluted (1: 1) HNO3 and evaporate to moist salts, which are then dissolved in water, filtered into a volumetric flask of 100ml. The copper content is determined with MPAMPT
Determination of copper in the gelatin: 5grams of gelatin in a porcelain dish soak 50ml of distilled water for 2-3hours. By the swollen gelatin was added 25mL (1: 1) HNO3 and heated in a boiling water bath for 2hours. The solution was filtered and neutralized with NH4OH (1:1) was transferred into a volumetric flask of 50ml. In solution, the copper content is determined with MPAMPT as well as dyetyldytyokarbamat
The reagent solution in chloroform has a yellowish color. The maximum light absorption is observed at 370nm. Structure of ligand was confirmed by using IR spectra.18‒20 IR (КBr): 3410 см-1 ν (NH), 3045 см-1 ν(CH), 2565см-1 ν(SH), 2968 и 2875 см-1 ν(-CH3), 1570см-1 δ(C6H5), 1380 см–1 δas (-CH3). The chemical structure of the reagent is shown in (Figure 1). Cu(II) reacts with MPAMPT and gives a blue colored complexes. These complexes are soluble in non-polar solvents.
The choice of the extractant
The extraction of the complex has been tried with several solvents: chloroform, 1, 2-dichloroethane, tetrachloromethane, dichloromethane, benzene, chlorobenzene, toluene, xylol, butanol, isoamyl alcohol, cyclohexane, ethyl acetate, isobutanol, isoamyl acetate and their mixes. Extractibility of complexes was estimated in coefficient of distribution and extent of extraction. Thus basicity of amines has no noticeable impact on conditions and extraction of complexes. Fast division of layers and the maximum value of molar coefficient of absorption were received at extraction of complexes by chloroform. After a single extraction with chloroform, 97,6% of copper was extracted as an colored complex (in a case the dichloroethane and carbontetrachloride was removed 95,7% of Copper). Further researches were conducted with chloroform. The concentration of Copper in the organic phase was determined with rubeanic asid21,22 by photometric measurements afterback extraction, while in the aqueous phase it was determined by the difference.
Extraction as a function of pH
Change in pH affected the complexation of Cu(II)-MPAMPT. Therefore, the absorbance of complex was studied between pH 1 to 10 by using dilute HCl and NaOH solutions. The absorbance values of extracted complex were measured. The maximum absorbance was obtained in the pH range 5.4 to 6.8 (Figure 2). Beyond this pH range, the observed absorbance values were lower. Thus further extraction and determination carried out at 6.
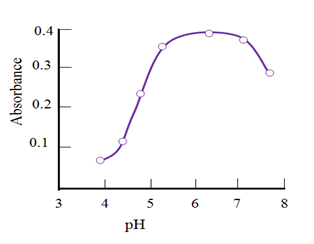
Figure 2 Absorbance of mixed-ligand complexes as a function of the pH of the aqueous phase = 1.875 ×10-5 М; С MPAMPT =5.0×10-4М, -2, =590 nm, l=0.5 cм.
Absorption spectrum
1-(2-Metoxiphenylamin)-3-metoksipropanthio l-2 forms a sparingly soluble complex with copper (II). The complex can readily be extracted quantitatively into chloroform in the pH range 5.4–6.8 absorption maximum in the visible region at 605nm (Figure 3). The reagent has a negligibly small It is evident from the spectrum that the blue solution of the complex in chloroform shows an absorbance at the of the complex and, hence, does not interfere with the determination of copper. Thus, further absorbance measurements of the complex were made at 590nm.
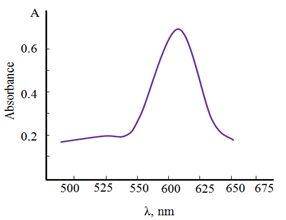
Figure 3 Absorption of complex Cu – MPAMPT =1.875 ×10-5 М; С MPAMPT =5.0×10-4М, Shimadzu UV1240, l=1cм.
Effect of reagent concentration and of shaking time
For the formation and extraction of complex, a 10-15-fold excess of complexing reagent is required; for example, theoptimal conditions for formation and extraction of these compounds are provided by 5.0×10-4 M MPAMPT. However it was found that the presence of excess of the reagent solution does not alter the absorbance of the color reaction. Under optimum conditions the absorbance of the complex formed in the aqueous phase after equilibration with toluene increased initially and then achieved a constant and maximum value for 10-180s. Therefore, 30s was selected as the optimum equilibration time for each extraction during further studies and has been used in the proposed procedure. Based upon the above study optimum conditions providing maximum, stable and reproducible absorbance values were selected and incorporated in the proposed procedure.The equilibration time of 3.0 minute is sufficient for the quantitative extraction of Copper. The stability of colour of the Cu(II)-MPAMPT complex with respect to time shows that the absorbance due to extracted species is stable up to 36hours, after which slight decrease in absorbance is observed.
Stoichiometry of the complexes and the mechanism of complexation
The stoichiometry of the Cu(II): MPAMPT complex was determined by Starik-Barbanel relative yield method, equilibrium shift method, crossed lines method and Asmus’ methods.23 It shows that the composition of Cu(II): MPAMPT complex is 1:2 (Figure 4). The probable structure of the complex was supported by the IR spectra, in which absorption bands in the 3250-362cm-1 with a maximum at 3475sm-1 observed in the spectrum of MPAMPT, says that the -NH group is involved in the formation of the complex (Figure 4). The observed decrease in the intensity, absorption bands in the area 2580sm-1 shows that the -SH groups involved in the formation of coordination bond. The (C-N) bands occur as a sharp peak in the ranges 1421 - 1431cm-1. Detection of the absorption bands at 1410cm-1 indicates the presence of a coordinated -NH group.18‒20 New bands were observed between 400-600cm-1 region in the complex, which were absent in the spectrum of ligand. The bands between 455сm-1 were assigned to stretching frequencies of υ(Cu-S) and the band between 575cm-1 have been assigned to the stretching frequencies υ(Cu-N) respectively. The iron content in the complexes was determined after their decomposition aqua regia photometrically using phenantroline. The purity of the compound was checked by the elemental analysis. Elemental analysis individually complexes are Table 1.
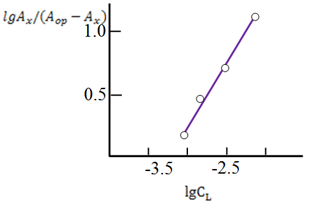
Figure 4 Determination of the ratio of components by equilibrium shift method for Cu-MPAMPT 1.875 ×10-5 М; С MPAMPT =5.0×10-4М , pH=6, λ=590 nm, KFK-2. ℓ=1.sm.
Compound |
% |
C |
S |
N |
Cu |
L |
Found |
58.55 |
14.1 |
6.25 |
- |
Calculated |
58.41 |
14.16 |
6.19 |
- |
|
Cu-L |
Found |
51.21 |
12.48 |
5.37 |
12.48 |
Calculated |
51.16 |
12.4 |
5.42 |
12.4 |
Table 1 Elemental analysis of ligand L and complex Fe-L.
Thermogravimetric study of the complex Cu- MPAMPT shown that thermal decomposition of the complex takes place in two stages: at 60-110оС water evaporates, at 430-500оС-decomposed MPAMPT. The final product of the termolysis of the complex is CuO. The stability constant of complex Cu(II)- MPAMPT was calculated and found to be lgβ=12.84 at room temperature. The sizes of equilibrium constant K ecalculated on a formula lgKe= lg D-lg [Аm] were presented in Table 4. Additional experiments by the Akhmedly’s method24 showed that the complex exists in monomeric form in the organic phase (the obtained coefficient of polymerization γ was equal to 1.08). In conclusion the analytical parameters pertaining to the proposed method are given in Table 4. It was found using the Nazarenko method that Cu(II) in the complexes was present in the form of Cu2+. The number of protons replaced by cobalt in one MPAMPT molecule appeared to be one.25,26
Influence of foreign ions
To evaluate the complex applicability for photometric determination of copper, we examined the influence of foreign ions and reagents.The tolerance limit of the ions shows minimum deviation (±2%) in absorbance. Influence of a number of cations and anions on the accuracy of determination of Cu (II) was studied. Experiments were performed according to the recipe, by which established Calibration curves, with the only difference that a solution other than Cu (II) injected a certain amount of the corresponding ions. The interference of various cations was removed by using suitable masking agents. The ions which show interference in the spectrophotometric determination of Copper were overcome by using appropriate masking agents. The effect of various ions and reagents on the extraction-spectrophotometric determination of 30mg copper (II) is summarised in Table 2. It can be assumed that large amounts of alkaline ions, alkaline-earth ions, CI-, . Tartrate, citrate, F- , J-, CN-, thiourea interfere determination of Cu (II). Co (II). N i(II), Fe(II, III), V(IV,V), W(VI), Mo(VI), Ti(IV) and Mn (II) interfere determination of Cu(II). However, the interfering effect of some of these ions can be reduced by masking with oxalate, citrate or EDTA. Interference of Fe(III) eliminated oxalic acid; Ti(IV) - tiron or sodium fluoride; Hg (II) ion -sulfit; Nb (V) and Ta (V) - oxalic acid, and Mo(VI) and W(VI) - sodium fluoride and oxalic acid. When using a 1% solution of ascorbic acid does not interfere with determination Mn (VII), V (IV), Nb (V), Cr (VI), Mo (VI) и Fe (III). When using 0.01M oxalic acid definition not interfere V (IV), Nb (V), Ta (V), Cr (III), Mo (VI), W (VI) и Fe (III). The proposed method compares favorably with the existing ones (Table 3) and offers the advantages of better simplicity, rapidity, sensitivity and selectivity.
Ion |
Molar Excess of the Ion |
Masking Agent |
Found Cu, µg |
Sr |
Co(II) |
60 |
30.1 |
0,05 |
|
Ni(II) |
60 |
Sodium cyanide |
29.8 |
0,03 |
Fe(II) |
35 |
29.8 |
0,04 |
|
Cd(II) |
250 |
29.6 |
0,05 |
|
Al(III) |
200 |
29.7 |
0,02 |
|
Fe(III) |
40 |
Sodium Fluoride |
30.5 |
0,05 |
Zr(IV) |
50 |
29.8 |
0,03 |
|
W(VI) |
45 |
29.8 |
0,04 |
|
Hg(II) |
38 |
Na2S2O3 |
30.2 |
0,05 |
Ti(IV) |
60 |
Sodium Fluoride |
29.8 |
0,04 |
V(IV) |
30 |
29.8 |
0,06 |
|
Mo(VI) |
20 |
Citrate |
30.5 |
0,04 |
Cr(III) |
120 |
30.4 |
0,04 |
|
Nb(V) |
50 |
Sodium Fluoride |
30,3 |
0,03 |
Ta(V) |
50 |
Sodium Fluoride |
29.5 |
0,04 |
Pb (II) |
25 |
30.3 |
0,05 |
|
Pd (II) |
10 |
29.2 |
0,04 |
|
Pt(II) |
10 |
30.4 |
0,05 |
|
Ag (I) |
25 |
Potassium iodide |
30.2 |
0,05 |
|
50 |
29.5 |
0,06 |
|
Bi(III) |
50 |
30.5 |
0.05 |
|
Acetate |
110 |
30.2 |
0,05 |
|
Tartarate |
110 |
30.3 |
0,05 |
|
Sulphate |
125 |
29.9 |
0,02 |
|
Thiourea |
25 |
29,7 |
0,05 |
|
Fluoride |
110 |
30,5 |
0,06 |
|
Thiosulphate |
36 |
30.2 |
0,04 |
Table 2 Influence of interfering ions on the determination of copper (II) with MPAMPT (30.0 µg Cu added.
Reagent |
Ph (Solvent) |
l, nm |
e×1 -4 |
4-11(carbon tetrachloride) |
436 |
1.4 |
|
4-7(isoamyl alcohol) |
546 |
0.64 |
|
3-10(isoamyl alcohol) |
454 |
0.79 |
|
DTMP+Phen |
6.7-7.9 (Chloroform) |
630 |
3.45 |
DTMP +BPhen |
6.6-8.1 (Chloroform) |
635 |
4.37 |
DTMP +Dip |
6.5-7.7 (Chloroform) |
629 |
3.28 |
MPAMPT |
5.4-6.8 Chloroform) |
605 |
4.32 |
Table 3 Comparative Characteristics of the Procedures for Determining of Copper.
Beer’s law and analytical characteristics
The adherence to Beer’s law was studied by measuring the absorbance value of the series of solutions containing different concentrations of the metal ion. A linear calibration graph drawn between absorbance and the metal ion concentration indicates that Cu(II) may be determined in the range 0. 5-16µg/ml.27 The equations of the obtained straight lines and some important characteristics concerning the application of the ternary complexes for extractive-spectrophotometric determination of Cu (II) are listed in Table 4. The proposed method compares favourably with the existing ones (Table 3) and offers the advantages of better simplicity, rapidity, sensitivity and selectivity.27 In conclusion the analytical parameters pertaining to the proposed method are given in Table 4.
Parameter |
Value |
Color |
blue |
The pH range of education and extraction |
3.0-9.0 |
The pH range of maximum extraction |
5.4-6.8 |
lmax (nm) |
605 |
Molar absorptivity (L· mol-1 cm-1) |
4.32·104 |
Sandell’s sensitivity (ng·cm-2) |
1.48 |
R,% |
97.6 |
The equation of calibration curves |
0.025+0.041x |
Correlation coefficient |
0.9964 |
lg ke |
5.76 |
Stability constant (β) |
14.5 |
Beer’s law range (µg·ml-1) |
0.05-16 |
Limit of detection (LOD): ng ·mL |
13 |
Limit of quantification (LOQ): ng ·mL-1 |
42 |
Table 4 Optical characteristics, precision and accuracy of the spectrophotometric determination of Cu (II) with MPAMPT.
Application
The proposed method was successfully applied for the determination of copper from various pharmaceutical, food and in plant sample.The results found to be in good agreement with those obtained by the standard known method (Table 5) (Table 6).
Sample |
Method |
(Mg/Tablet) |
RSD (%) |
|
Zincovit (Apex) |
Diethyldithio carbamate |
0.5 |
1.21 |
0.50±0.007 |
MPAMPT |
0.51 |
1.21 |
0.51±0.007 |
|
Multi vitamin capsule (A-Z) |
Diethyldithio carbamate |
0.88 |
0.95 |
0.89±0.009 |
MPAMPT |
0.93 |
0.79 |
0.93±0.008 |
|
Revital (Ranbaxy) |
Diethyldithio carbamate |
0.49 |
1.27 |
0.49±0.006 |
MPAMPT |
0.52 |
1.15 |
0.51±0.006 |
|
Supradyn |
Diethyldithio carbamate |
0.98 |
0.92 |
0.98±0.009 |
MPAMPT |
1.03 |
0.85 |
1.03±0.009 |
Table 5 Determination of Cu (II) in Pharmaceuticals samples (n=3, P=0.95).
Sample |
Method |
µg/Kg |
S |
Sr |
|
Beans |
Rubeanic acid |
6,05 |
0,304 |
0,048 |
6,05±0,30 |
MPAMPT |
5,93 |
0,207 |
0,035 |
5,93±0,21 |
|
Gelatin |
Rubeanic acid |
12,10 |
0,508 |
0,042 |
12,10±0,53 |
MPAMPT |
11.74 |
0,305 |
0,026 |
11.74±0,32 |
|
Wheat bran |
Rubeanic Acid |
5,65 |
0,198 |
0,035 |
5,65±0,21 |
MPAMPT |
5,33 |
0,150 |
0,028 |
5,33±0,15 |
|
Rye |
Rubeanic acid |
4.3 |
0.1892 |
0.044 |
4.30±0.198 |
MPAMPT |
4.15 |
0.1618 |
0.039 |
4.15±0.170 |
Table 6 Determination of copper in food (mg / kg). n = 6, P = 0.95.
The results obtained show that the newly developed method in which the reagent MPAMPT was used, can be effectively used for quantitative extraction and estimation of Cu(II) from aqueous media. Complex of copper(II) with MPAMPT have been investigated by spectrophotometric method. Extraction of complex is maximal at pH 5.4-6.8. The proposed method is quick and requires less volume of organic solvent. The optimal conditions for the formation and extraction of compound have been found and the ratios of components I the complexes have been determined. The Beer’s law was applicable in the range of 0.5-16µg/ml. A simple, rapid and sensitive methods proposed for the determination of trace amounts of copper. The method is very precise, faster and simpler than other methods. The proposed method was successfully applied for the determination of copper from various pharmaceutical, food and in plant sample. The obtained by independent methods similar values of some of the mentioned above characteristics are the evidence for the correctness of the performed experiments.
None.
The author declares no conflict of interest.

©2017 Zalov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.