eISSN: 2574-9927


Mini Review Volume 6 Issue 4
1Nanochemistry Laboratory, Department of Chemistry, Jamia Millia Islamia, India
2Department of Chemistry, College of Science, King Saud University, Saudi Arabia
Correspondence: Tokeer Ahmad, Nanochemistry Laboratory, Department of Chemistry, Jamia Millia Islamia, New Delhi-110025, India
Received: December 07, 2022 | Published: December 20, 2022
Citation: Ubaidullah M, Fazil M, Ahmad T. Short review on fabrication, structural and dielectric characterization of zirconium based oxide nanoparticles. Material Sci & Eng. 2022;6(4):152-156. DOI: 10.15406/mseij.2022.06.00193
Zirconum based oxide nanoparticles of general formula Ba1-xPbxZrO3 and Ba1-xSrxZrO3with dopant concentrations ranging from 0 to 1 using reverse micellar and polymeric citrate precursor methods are discussed. Presently, hundreds of dielectric materials have currently been synthesized. Zirconium-based oxides are the significant class of dielectric materials among them because of their structural flexibility and simplicity. This review summarizes recent advances in zirconium-based oxide synthetic strategies, their significant use as dielectric materials for wireless communication devices such as electroceramics and piezoelectric devices, and efforts being made to alter their physicochemical properties and increase their efficiencies by adjusting reaction conditions. The article's goal is to describe approaches controlling these materials' efficacy and upcoming difficulties for practical applications.1–3
Keywords: Nanoparticles; Zirconium oxide; Solid solution; Reverse micelle; Polymeric citrate precursor method; Dielectric properties
Nanoparticles are of particular scientific importance because they provide a connection between bulk materials with atomic or molecular structures. These show highly unusual properties and are widely used for numerous applications, especially in mechanical, electrical, photochemical, catalysis, protective coatings, effective antibacterial, water purification, high energy storage devices, and dynamic random access memories (DRAM). Nowadays, functional nanostructures find applications in nanocatalysis for hydrogen generation.4–8 BaZrO3 and SrZrO3 are two zirconium-based dielectric oxides that have attracted much interest due to their special features for this application9,10 in multilayer ceramic capacitors (MLCC), piezoelectric and pyroelectric sensors, heterogeneous catalysts, wireless communication devices and refractories.11–14 The easiest and most interesting way is the polymeric citrate precursor method, which provides greater stoichiometry control, lower synthesis temperatures, and simple dopant introduction.15 The approach's fundamental principle is to use in-solution polymerization of monomers that have been an addition to the necessary metal cations. This procedure is called the Pechini method.16 The backbone of this method is the polyesterification reaction between ethylene glycol and multifunctional carboxylic acid. The solution provides the conditions for preventing cation segregation during the earliest stages of polymer formation. Afterward, the rather rigid polymer network traps cations and maintains the original homogeneity of the solution. For the synthesis of Zirconium-based dielectric oxide, the microemulsion is also an important method for various simple and complex materials.17–26 It was reported that grain size and material morphology can be easily manipulated at the nanoscale level using a modified reverse micellar technique. The main objective of this article is to shorten the gap between research and applicability of zirconium-based oxides by upgradation in the fabrication routes, and physicochemical properties towards dielectric applications.
Scope of the review
While there is enough literature on the Zirconium-based oxide nanostructures, none of them provide a systematic overview of the field, including its synthesis, structural design, and dielectric applications. In this literature, we have examined the many uses of nanostructured oxides of zirconium for dielectric applications with respect to dielectric oxides. In the later section, we have addressed the synthetic strategies that result in qualitatively and quantitatively promising products based on Zirconium-based Oxide nanostructures. This review is offered to provide a brief outline of the dielectric applications of these materials because they are underutilized in this field. In this review, we have provided the information that will pave the way for further exploration of their potential uses in fields such as material chemistry, nanochemistry, multilayer ceramic capacitors (MLCC), piezoelectric, wireless, and communication devices. In addition, we have reviewed, methods to broaden the range of zirconium-based oxides applications and their dielectric applications.
Polymeric Citrate Precursor Method (PCP)
The PCP is a temperature synthesis route for nanomaterial oxides, also known as the amorphous complex method.2,14,16,27 The Pechini patent16 on making thin film capacitors by using multifunctional organic acids that can trap metal ions into stable complexes and a diol, which acts as a solvent during the complex formation step and then takes part in the polyesterification reaction to create a three-dimensional polymer network with mixed metal ions on the atomic scale.28–31 The schematic representation of the reaction scheme is shown below in Figure 1. Citric acid and ethylene glycol are best suited for the reaction. Citric acid is a relatively strong multifunctional organic acid. Its structure facilitates the formation of five or six-membered rings in the metallic citrate complexes, known as polymeric matrix or cavity. The chemical reaction hence takes place inside this matrix. The chemical reaction between ethylene glycol and citric acid takes place at room temperature, without applying thermal energy. It is well known that the boiling point of ethylene glycol is the lowest among all the diols. As a result, using ethylene glycol as a solvent and a monomer proved extremely convenient. Formation of metallic citrate complex and then its conversion into the polymeric precursor form,as shown in Figure 2. Polymeric citrate precursors were used to fabricating nanocrystalline solid solutions of Ba1-xSrxZrO3 and Ba1-xPbxZrO3 (0£ x £1).30,32
The fabrication of thin film capacitors using the sol-gel PCP method described in the original patent could be one of the reasons for the 1:4 CA/EG ratio. In this case, the film's quality will be heavily influenced by the gel's viscosity, and to get a high-quality film, foaming during polymer pyrolysis must be fully avoided. The range of CA concentrations from 50 to 60% appears to be the most ideal for creating complex oxide powders, as shown in Figure 3 because it gives the highest viscosity of the resulting gel. Strong foaming is another element that inhibits segregation during polymer heat degradation.
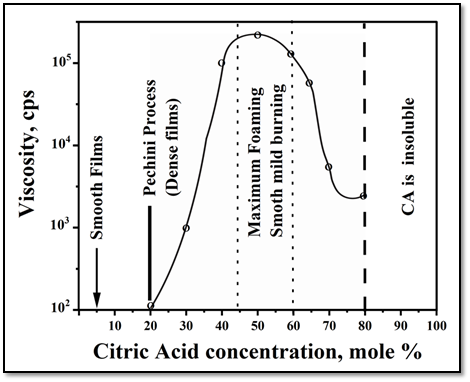
Figure 3 Effect of CA/EG ratio in the starting solution on the gel properties in the Pechini Method.
Advantages of polymeric citrate precursor method
Applications of polymeric citrate precursor method
Applications of the PCP method are based on outstanding homogeneity of the metal ions distribution in the solution, reducing synthesis temperature because of a shorter diffusion path, formation of nanoscale powders after gel pyrolysis, the liquid state of the polymer solution whose viscosity can be easily adjusted, or on various combinations of these advantages depending on specific material composition and its intended applications. Due to the low cost of precursors, low synthesis temperature, and ionic homogeneity attained at the molecular level, this method is widely used for synthesizing complex pure multicomponent oxide materials, including ferromagnetic ones, semiconducting, ferroelectric, photocatalytic, andfuel cell, respectively. The PCP approach is of enormous practical value in low-temperature processing that can be efficiently applied for the synthesis of oxide materials containing volatile components such as alkaline oxides Bi2O3 and PbO. Also, the synthesis of oxide materials at reduced temperatures enables and the low-temperature part of phase diagrams when we cannot achieve equilibrium in a reasonable time scale using conventional solid-state reactions.
Another important application of this method is the homogeneous distribution of a small number of dopants. This is the reason for success in the reproducible synthesis of phosphor materials with high luminescence intensity. Also, the homogeneity on the atomic scale tremendously decreases the diffusion paths. Consequently, most multi-component oxide materials are formed directly after the pyrolysis of the polymer decomposition of the carbonates. Because of the slow grain formation at low processing temperatures and the foaming of the polymer during the evolution of the gaseous products during pyrolysis, this technology is highly beneficial for synthesizing catalytic materials for organic chemistry and photocatalysis.
Reverse micellar method
A surfactant stabilizes water-in-oil droplets to form reverse micelles. These droplets are randomly dispersed and subjected to Brownian motion. The polar head groups of the surfactants attract the aqueous phase and point inwardin a typical reverse micelle configuration (Figure 4). In contrast, the hydrocarbon chain of the surfactants attracts the oil phase and points outward.
Reverse micellar synthesis occurs when reactions occur among reactants only available inside the micelle, and the particle stops developing after the reactants are consumed. Surfactants preferentially self-assemble at the air/aqueous or hydrocarbon/aqueous solution interfaces when dissolved in solvents. The hydrophilic portion is directed toward aqueous solutions. Surfactants self-assemble to create micelles when their concentration reaches a certain level, this minimum concentration of surfactant at which the micellization process occurs is called critical micellar concentration (CMC). The dispersion of fine liquid droplets form organic solution and water is known as a microemulsion. A microemulsion technique of this type can be utilized to synthesize nanoparticles.33,34 Microemulsions are isotropic, macroscopically homogeneous, and thermodynamically stable solutions made up of at least three components: a polar phase (often water), a nonpolar phase (typically oil), and a surfactant.The mechanism involving particles formation through microemulsion is given in Figure 5, which clearly shows the inter-change of the matter/inter micellar exchange.
Numerous nanosized dielectric oxides such as BaTiO3,35 Ba2TiO4,36 SrTiO3, PbTiO3 and Sr2TiO4,37 SrZrO3,38 and (Ba,Pb)ZrO3,39 have been successfully prepared and reported by reverse microemulsion method. These reports also demonstrate the detailed characterization and dielectric properties of the above dielectric oxides.
Surfactants are molecules made up of hydrophobic (hating) and hydrophilic (loving) components (water-loving).These are surface active agents or amphiphiles that can be adsorbed at the interface of two immiscible phases, such as air-water or air-oil, to produce an oriented monolayer with hydrophilic groups pointing towards the watery phase and hydrophobic groups pointing towards the air or the oil phase. During the adsorption of surfactant molecules, there is a net change in the interfacial tension. Based on the head group charge, surfactants are divided into four categories: i) Cationic, ii) Anionic, iii) Nonionic, and iv) Zwitterionic. The anionic surfactant dissociates in an aqueous solution, yielding an anion with amphiphilic characteristics and an inactive cation (e.g., Na+ or K+). In aqueous solutions, cationic surfactants ionize into an amphiphilic cation and an inactive anion, such as Cl- or Br. Most cationic groups are quaternary ammonium groups. As the name implies, nonionic surfactants do not produce ions in solution. Polar groups such as ether, alcohol, carbonyl, or amino groups are found in the hydrophilic portion of their molecule. Ionic surfactants with positive and negative charges on the same molecule are known as amphoteric surfactants.
After forming a rough emulsion, a co-surfactant is added, and the system is titrated to clear. When the four components are properly combined, the system cleansed itself. These systems were spherical microdroplets ranging from 5 to 20 nm. Co-surfactants are used in conjunction with ionic surfactants like medium chain-length alcohol. Because the co-surfactant is an uncharged entity, the electric field does not affect its adsorption. As a result, it offers the extra interfacial tension reduction required for microemulsion production. Co-surfactants are often alcohols or amines with C4 to C10 carbon atoms that aid in creating and stabilizing micelles/microemulsions. It also serves as an organic solvent in several circumstances. The co-surfactant provides a "dilution effect" in addition to the surfactant and causes a further decrease in the interfacial tension. Co-surfactants' amphiphilic nature may also allow them to diffuse between the aqueous and oil phases.40 For the utmost use of microemulsion for the synthesis of nanomaterials, a complete understanding of the pseudo-ternary phase diagram comprising oil /surfactant/water/co-surfactant is necessary. With the help of titrimetric analysis, the microemulsion region can be conveniently traced out on a Gibbs'triangle.
Electrical properties
Zirconates are a class of materials that shows unusual dielectric properties due to which these materials attract the scientific community to a large extent. The main reason behind this it's large use in the electronic and communication industry. Therefore, the dielectric properties of Zirconates are of great significance. The alkaline earth Zirconates have the general formula MZrO3 where (M=Ca, Sr, and Ba). They all have ABO3 perovskite structure. It has been reported that they become ionic or electronic conductors on doping. These Zirconates are very useful in electronic ceramics. The corresponding titanates, such as BaTiO3 and SrTiO3 are well-known electro-ceramic materials.
The term dielectric was first coined by Faraday.41 Dielectric materials are electrical insulators; hence, they are widely used in capacitors and electrical insulators. Insulators are bad conductors of electricity. A dielectric material can be polarized by an electric field because when an electric field is applied the electrical charges shift from their average equilibrium position. Positive charges move along the field, whereas negative charges move in the opposite direction. A dielectric material should have high dielectric strength and low dielectric loss. They should not undergo degradation on applying high voltage and should have a minimum loss of electrical energy appearing in the form of heat. The behavior of the material may define dielectric properties by the material's behavior in parallel plate capacitors. The capacitor is a pair of parallel conducting plates separated by a medium (Figure 6).
This medium is called 'dielectric'. The value of the capacitance between the plates C1 is given by the equation:
Where A is the area of the plates, d is the separation between the plates and ε (Greek letter epsilon) is the absolute permittivity of the dielectric, which is a measure of the electrostatic energy stored within it and therefore dependent on the material.
Since, ε =ε0εr
Where ε0 is the permittivity of free space (that is, of a vacuum), which has a value of 8.85×10-12 Fm-1, and εr is the relative permittivity, more usually called the "dielectric constant". BaZrO3 is a ferroelectric material. Ferroelectric materials are special dielectric material that retains residual polarization of charge even after the removal of the electric field. Thus, those materials that have remnant polarization are called ferroelectric materials, known as ferroelectricity. The polarization in ferroelectric materials is well explained by the hysteresis loop as shown in Figure 7.
The manuscript is summarized in the following points:
TA thanks UGC, New Delhi, Govt. of India for Research Grant for In-service Faculty Members. Authors also thank SERB, CSIR and MoE (SPARC/2018-2019/P843/SL) for financial support to Nano Chemistry and Nano Energy Labs. MF thanks to UGC, New Delhi for the research fellowship.
None.
There are no conflicts of interest.

©2022 Ubaidullah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.