eISSN: 2574-9927


Research Article Volume 7 Issue 3
1University Ss Cyril and Methodius in Skopje – Faculty of Technology and Metallurgy Macedonia
2Department of Chemistry, Hacettepe University, Turkey
3Institute of Public Health of Republic of Macedonia, Macedonia
Correspondence: Anita Grozdanov, University Ss Cyril and Methodius in Skopje – Faculty of Technology and Metallurgy, Rugjer Boskovic 16, 1000 Skopje, Macedonia
Received: September 27, 2023 | Published: October 12, 2023
Citation: Grozdanov A, Paunovic P, Dimitrievska I, et al. Electrochemical detection and evaluation of diclofenac chloride with modified screen printed electrodes.Material Sci Eng 2023;7(3):158-164. DOI: 10.15406/mseij.2023.07.00219
Last decade, due to the safety concerns, the problem of detection of the low level of various drugs including the anti-inflammatory drugs (AIDs) in the waters became very important. Electrochemical methods offer an acceptable level of detection providing low-cost, reagent-consuming and fast analysis. So, in this study, a Cyclic Voltammetry (CV) was discussed in detail, along with scanning electron microscopy (SEM) and atomic force microscopy (AFM), as analytical characterization techniques. Screen-printed electrodes (SPE) based on carbon nanotubes (CNTs) were explored as a biosensors for ultrasensitive detection of biochemical entities, such as active pharmaceutical ingredient (API) – Diclofenac chloride (DIC). Surface modification of the SPEs was done using Polyvinylidene fluoride (PVDF), applied with drop modification method. Physical characterization made with SEM and AFM has found the surface morphology and grain topography of the working electrodes as well as the roughness changes. Results obtained with the electrochemical analysis show that the acidic environment contributes for better redox processes hence modifications with PVDF show better electrochemical response at low pH. In terms of redox processes and pH influence, diclofenac shows strong electrochemical response.
Keywords: biosensors, Screen-printed electrodes, carbon nanostructures, PVDF, drop modification, diclofenac chloride, cyclic voltammetry
Screen-Printed Electrodes (SPE) allowing rapid in-situ analysis were extensively used in modern biosensor applications. A Cyclic Voltammetry as an electroanalytical technique offers one of the most promising approaches towards simple and low-cost commercial availability of biosensors. SPE gained popularity and have been an interesting focus as disposable electrochemical devices emerging not only in amperometric detection but also in voltammetric measurements,1 due to their fundamental properties like versatility, robustness, high reproducibility, selectivity, and perhaps the most crucial of all, miniaturization.2,3
The development of nanosensors, especially the application of carbon nanostructures in screen-printed electrodes, opens a wide possibility for modification and improvement of their electrochemical performance. Modified CNTs exhibit interesting electrochemical behavior, due to the presence of reactive functional groups on the nanostructure’s surface.4 Appropriate alteration of the surface includes addition of various metals, enzymes, complexing agents, polymers etc., thus creating an excellent system for detecting bioactive components, drug dosing, gene therapy, biological imaging, antibacterial applications, cell cultures, tissue engineering and biosensors. 5–8
Disposable nanosensors could be particularly used for pharmaceutical analysis in various matrices working as immunosensors in quality control, residues determination, pharmacokinetic studies, and drug monitoring.9,10 However, one of the main problem when considering conventional electrochemical sensory systems are electrode “fouling” or passivation that occurs in pharmaceutical formulation analysis, in which both matrix components and pharmaceuticals can absorb on to the electrode surface, altering the measurement and hence reducing the sensitivity of the method.11,12 There are numerous related research efforts of the development and modification biosensors based on SPE and accordingly there is a considerable amount of valuable literature and reviews focusing the horizon on the pharmaceutical application.3,12–14 Their application in drug analysis is one of the most active areas of research in analytical chemistry.15
Biosensors’ development began in the early sixties with the concept of enzyme-immobilized electrodes used for determination of physiological analytes such as glucose and urea.16 Improved biosensors based on conversion of physiological components by a biocomponent, attached near physical transducer17 were constructed for assay analysis of glucose, urea, lactate etc.18,19
Due to the low background current, renewable surface, and wide potential window, carbon nanotubes are commonly used in construction of SPE.12 Sarakbi et al., proposed an electrochemical detection method of analgesic drug acetaminophen and ascorbic acid (Vitamin C) based on liquid chromatography20 using a carbon SPE.21 Frag et al., reported comparison between biosensors based on SPE and carbon paste electrodes used for potentiometric quantification of ocular vasoconstrictor naphazoline hydrochloride in pharmaceutical formulations.22 Furthermore, the same authors studied the application of both SPE and carbon paste electrodes for determination of antihistaminic diphennydramine hydrochloride in pharmaceutical formulations and biological fluids i.e., blood serum and urine.23 Mohammed et al. reported screen-printed carbon ink-based biosensor used for rapid analysis of antiseptic Septonex-tetraphenylborate without prior separation or derivatization.24 Bergamini et al. proposed biosensor based on poly-L-histidine film coated electrode for voltametric detection of antituberculosis drug isoniazide. Recently, Abbas et al., reported gallamine-modified carbon paste electrode based on gallamine–tetraphenylborate as electroactive material for determination of gallamine triethiodide, a muscle relaxant drug.24 Felix et al., reported carbon film electrodes employed as sensors in electrochemical determination of ambroxol in different pharmaceutical samples, without any sample pretreatment.25 The same authors proposed electrochemical determination of acetaminophen, also known as paracetamol, in different pharmaceutical tablets using carbon film electrodes, without any sample pretreatment or electrode modification being necessary.26
Diclofenac chloride (DIC) belongs to the group of the nonsteroidal anti-inflammatory drugs used for the treatment of pain and inflammation. In the same time, diclofenac can also provoke some serious effects on the stomach and intestines, including bleeding and perforation. Besides that, there is environmental concern. Namely, it was registered that in Germany alone 90 t were used annually, of which 63 tons entered the waste water after being excreted in urine.3 The exceptional use of DIC and its release into the environment may cause risks to human health and aquatic environments. Because of these facts, fast and low-cost detection of DIC became very important. Besides the conventional methods (gas chromatography, spectrophotometry, spectro- fluorometry) used for detection of DIC, last few years some alternative methods such as the electrochemical methods were considered due to its high electroactivity.27–29 Electrochemical methods offer some advantages, for example high sensitivity, specificity, low detection limit (LOD), low cost, user-friendly operation and simplicity. Boumya and his team reported an comprehensive review of the most promising nanostructures and organic molecules used for the electrochemical and biosensing of DCF.29
The aim of this study is to develop a novel biosensor based on non-covalent modification of CNTs-coated SPE with Polyvinylidene fluoride as well as to pursue an analytical procedure for determination of diclofenac chloride in pharmaceutical formulations. Polymer-modified nanostructures were obtained by drop modification method on the surface of SPEs and further characterized with various analytical techniques.
Chemicals, solutions, and pharmaceuticals
Commercially available, carbon SPEs, based on multi-walled CNTs (model DRP110) with carbon working electrode of 4 mm diameter, were ordered from Dropsens Ltd, Llanera, Asturias, Spain, and further modified with corresponding polymer in order to increase the electrode sensitivity. Polyvinylidene fluoride (PVDF), dissolved in n-dimethylacetamide (Sigma-Aldrich Co.) at 2% concentration, was deposited on the surface of commercial SPEs.
Application of the sensors was studied by analyzing commercial diclofenac chloride formulations. Pharmaceutical samples of Diclo Duo (Pharma Swiss) were purchased from local pharmacies. The electrolyte samples were prepared by using DIC diluted in double distilled water (conductivity ≤ 0.1 µS.cm-1) and then the corresponding aliquot was mixed with phosphate-buffered solution. The final PBS concentration for kinetic analysis was selected to be pH = 7.4 simulating the pH of human body. All reagents and solvents were of analytical grade and used without further purification.
Modification and characterization
Experimental work in this research was carried out in three phases: modification, electrochemical and physical characterization.
The modification was done using drop modification method, where previously prepared polymer solvent of PVDF was applied on working electrode of commercial sensor’s SPE electrodes. After application, the polymer modified SPEs were dried at room temperature.
Further, the electrochemical behavior of the commercial and modified SPEs was followed by cyclic voltammetry (CV). Experimental scheme setup is shown on Figure 1. The electrochemical measurements were performed at room temperature, using the potentiostat-galvanostat SPELEC - "DROPSENS" from Spain, controlled by the software package Dropview. Experimental conditions for CV were optimized as followed: room temperature 23-25°C, number of scans 5, range (Evtx1 and Evtx2) –0.6 to +0.6 V, current automatic, Estep 0.02 V. Different scan rates were applied: 20, 50, 100, 200, 300 and 400 mV.s-1, in order to determine kinetics of the oxidation process and double layer capacity of the electrodes. The tested electrodes were immersed in different environments in order to monitor the pH effect on the sensors’ performance: phosphate-buffered saline (PBS) + solution of diclofenac chloride (DIC) in PBS at pH=3, 4.5, 5.5, 6.8, 7.4, 8.5 and 11.3.
All the obtained data were analyzed with Origin 8.5 software. Physical characterization on commercial and modified SPEs, was performed by scanning electron microscopy (SEM) and atomic force microscopy (AFM). Surface morphology of the SPEs was analyzed with FEI Quanta 2000 SEM, using a secondary electron detector and acceleration voltage of 30 kV, under high vacuum. AFM analysis was performed on a Veeco Multimode™ V scanning probe microscope (Veeco Metrology LLC, Santa Barbara, CA) using Nanoscope® 9.1 controller at tapping mode in air.
The electrochemical behavior of the commercial and PVDF-modified SPEs was studied in order to evaluate the response of tested drug diclofenac chloride (DIC), electroactive surface area of the working electrode and kinetics of the oxidation reactions of DIC, followed by cyclic voltammetry (CV). It is well known that the effect of pH of the electrolyte medium is important condition for redox processes and the response of the electrodes. Because of that, experimental optimization was prior performed in different pH conditions in range of pH from 3 to 11.3.
The cyclic voltammogram is a type of “electrochemical spectrum”, from which surface redox reactions can be determined that occur on PVDF/CNTs electrodes in interaction with the electrolyte – an aqueous solution of PDS and DIC. Figure 2 shows the cyclic voltammograms of the PVDF modified SPEs in wider pH range from 3 to 11, respectively. In cyclic voltammetry, achieving a rectangular diagram is considered a success and higher energy densities are expected due to the wider range of potentials that can be used. Higher current density is also expected by increasing the scan rate. When the diagram has such a regular rectangular shape, without certain distortions, then it is considered an excellent candidate for obtaining electrochemical double layer capacitors. Acidic environment (pH = 3) causes weak oxidation peaks at 0.26 V and 0.09 V with corresponding reduction peak at –0.1 V. Also there is a cathodic reaction peak at –0.24 V with corresponding anodic reaction peak at –0.28 V. In electrolyte with pH = 4.5 the firstly mentioned redox pair is shifted to negative values, while the second redox pair to positive values. The second redox reaction is completely reversible. The voltammograms obtained in pH = 5.5 and 6.8 did not give well shaped and pronounced redox reaction peaks. The voltammograms obtained at pH = 7.4 and 8.5 are very well shaped and intense. The redox peaks are shifted to positive values related to those obtained in more acid media. In both voltammogram, another (third) oxidation peak was detected near at 0.03 and 0.05 V, respectively. According the literature, the appearance of new peaks at higher pH could be explained with the electrochemical oxidation of hydrolytic products.30 The voltammogram obtained at pH = 11.3 is similar to those obtained in highly acidic media, but the corresponding redox reaction peaks are shifted to positive values and the current response is very higher. Due to the higher current response, the voltammogram scanned in electrolyte with pH = 11.3 is shown separately in the inset of Figure 2. In Figure 3 is illustrated the change of the current response (current at diclofenac chloride oxidation peak), which shows “Volcano” dependence. Because of the very well-shaped voltammogram and satisfying current response, the electrolyte with pH 7.4 was used for further electrochemical characterization (simulating also pH of human blood).

Figure 3 Change of current intensity of the firs oxidation peak (Pa1) determined from voltammograms in Figure1.
The anodic peak denoted as Pa1 apeears at 0.42 V for PVDF/CNTs electrode and 0.385 V for CNTs electrode, and corresponds to oxidation of diclofenac chloride to ion state (amine oxidation) with exchange of one electron, leading to subsequent hydrloysis.30 The steps in electrochemical oxidation of DIC are shown. For both electrodes this anodic reaction is irreversible, which is in accordance with literature data.30 he final step of DIC oxidation – transformation of C=O to C-OH followed with exchange of one electron, is identified with anodic peak Pa2 positioned at 0.26 V for PVDF/CNTs electrode and 0.165 V for CNTs electrode. Its related cathodic peak, Pc2 is positioned at 0.001 V for PVDF/CNTs electrode and 0.087 V for CNTs electrode. For CNTs electrode, the difference of the electrode potential of this redox pair (DE » 77 mV) is close to the Nernstian 59 mV, which points out on the reversibility of this process.31 For PVDF/CNTs electrode this difference is much higher (DE » 77 mV) and this process is far of reversibility. In both voltammograms, a residual anodic peak, weak and not well shaped can be seen at position of –0.14 V for PVDF/CNTs electrode and –0.02 V for CNTs electrode. This peak could be attributed to para-quinonic groups electro-generated at the graphite surface.30,31 After fifth cycle, it becomes more visible as shown in inset of Figure 4a. Comparing both voltammograms one can be seen that CNTs SPE shows better shaped, more rectangular spectrum with higher current response. This electrochemical response is due to the bonds between the CNTs atoms and its conductive bands, which make it a semi-metal with excellent electrical properties, and thus an excellent conductor of current. Also, CNTs have higher value of double layer capacity, i.e. higher reals surface area of the electrode. Addition of polymer (PVDF) over the CNTs surface reduces their surface area.
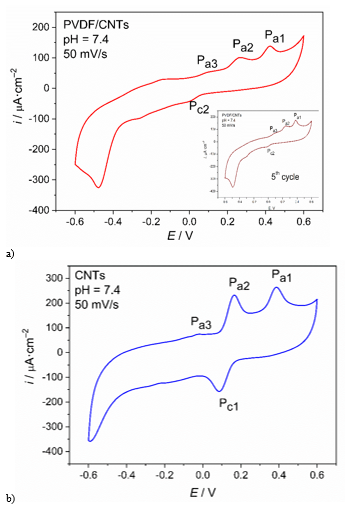
Figure 4 Cyclic voltammograms obtained at pH = 7.4 and scan rate of 0.05 V•s–1, for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
In order to determine the double layer capacity of the studied PVDF/CNTs and CNTs SP electrodes, cyclic voltammetry measurements were performed at different scan rates: 20, 50, 100, 200, 300 and 400 mV·s–1. The corresponding voltammograms of both systems are shown at Figure 5. As the scan rate increases, the current density of the all peaks increases. The corresponding potentials of the oxidation peaks shifted to anodic direction (more positive values of potential), while reduction peaks shifted to chatodic direction (more negative values of potential).
At the intersection of voltammograms in the region of charge and discharge of the electrochemical double layer (EC/D = 0 V for PVDF/CNTs and EC/D = –0.045 V for PVDF/CNTs), from the corresponding values of anodic and cathodic currents densities, the double layer capacity (Cdl) of the produced material can be determined, using the following equation 1:31,32
(1)
The current density of the electrochemical double layer icap.at given scanning rate, is determined as the mean value of the absolute values of the anode and cathode currents at the potential of the intersection of the curves at potential EC/D. The dependence of the changes of icap from scanning rate is a straightline, whose slope is the value of the double layer capacity, Cdl. This is presented in Figure 6. The accuracy of the obtained values of Cdl from the slope of the strightlines is very high. The calculated value for the double layer capacity of the PVDF/CNTs SP electrode is 0.72 mF·cm–2 (R2 = 0.9962), while the corresponding value for CNT SP electrode is about 2.5 times higher – 1.73 mF·cm–2 (R2 = 0.9948) (Figure 7).
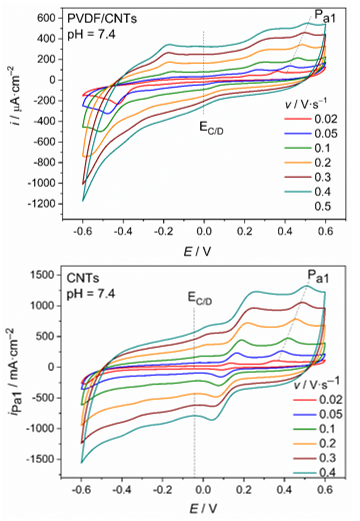
Figure 6 Cyclic voltammograms obtained at pH = 7.4 and different scan rate for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
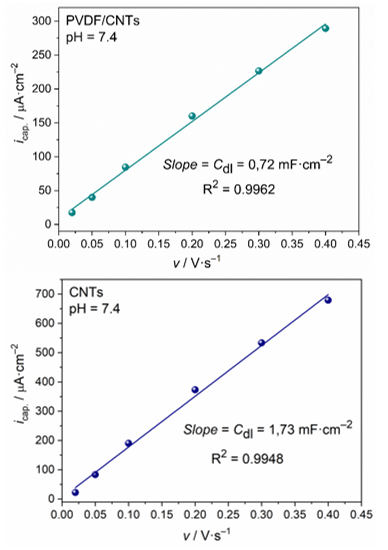
Figure 7 Linear fit of icap. – v dependence for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
By applying the polymer drop on the CNT surface, increased functionality and sorption pressure on the carbon nanotubes was expected. However, modification with PVDF-polymer drop on the CNT surface has decreased the surface roughness which was shown in AFM images presented in Figure 8.
The AFM images and the roughness values (Ra) calculated are presented in Figure 8. When the AFM image of the commercial CNT electrode is examined (Figure 8a), it is clearly seen that the carbon nanotubes with a diameter of about 31.3+/-2.1 nm are randomly distributed on the surface. After the addition of PVDF to the surface structure, the nanotubes are obviously covered by PVDF clusters to a large extent, in the form of speherical particles and the domains of PVDF appear to fill surround the nanotubes, resulting in a decreased in roughness from 16.7 nm to 9.78 nm (Figure 8b). Significant morphological changes detected by AFM analysis provide strong evidence confirming the modifications conducted and they are in agreement with the calculated value for the double layer capacity of pure CNT and PVDF/CNTs SP electrodes.
Cyclic voltammograms scanned with different scan rate can be used for kintetic analysis of the DIC oxidation process in order to discuss the reaction mechanism. Dependence of current density of the first oxidation peak Pa1 on the scan rate can be a criterion for the reaction mechanism, i.e. to determine limiting step of the DIC oxidation process. Straightline dependence of current density on the scan rate (iPa1–v) is criterion for adosption controled process, while the straightline dependence of current density on the squere root of the scan rate (iPa1–v1/2) is criterion for diffusion controled process. Shown in Figure 9 is the iPa1–v dependence for PVDF/CNTs and CNTs SP electrodes. For both electrodes this dependence is linear with high correlation (R2 = 0.98987 for PVDF/CNTs and R2 = 0.98812 for CNTs), indicating that adsorption process of the reacting species is limiting step of te oxidation reaction. Analyzing the second criterion, the iPa1–v1/2 (Figure 10) dependence for both electrodes was shown as strongly straightline (R2 = 0.99082 for PVDF/CNTs and R2 = 0.99338 for CNTs), highlighting on diffusion controled processes.
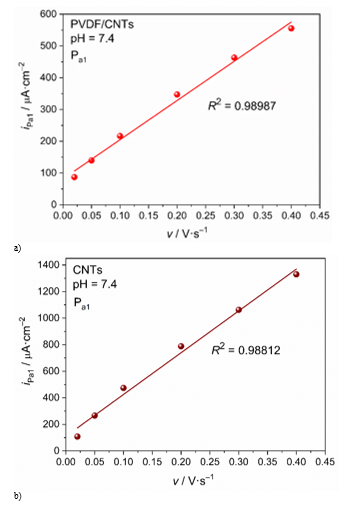
Figure 9 Linear fit of i – v dependence for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
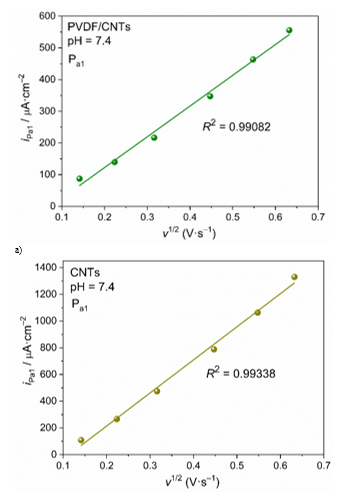
Figure 10 Linear fit of i – v1/2 dependence for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
The conclusions from both criteria are in contradiction. For this purpose, the most eligible sriterion is dependence of the logarithm of peak currents versus logarithm of scan rates (logiPa1–logv). The slope from this straightline depedence, is an indicator for the corresponding electrochemical reaction mechanism. The value of 0.5, points out on diffusion controled reaction, while the value of 1 is an indicator for slow adosrption of the reaction species on the electrode surface.33,34
logiPa1–logv plots for both electrode systems are shown in Figure 11. The straghtline dependence in the plot logiPa1–logv showed very high correlation for both electrode systems (R2 = 0.99568 for PVDF/CNTs and R2 = 0.99501 for CNTs). The slope of the straghtline for CNTs SP electrode is 0.825, which unequivocally indicates that the reaction rete determinig step is the adsorption of the reacting species on the electrode surface. The slope of the straghtline for CNTs SP electrode is 0.62. This value is close to 0.5, indicating that the oxidation rection is controled by diffusion.35–37 The application of a polymer layer (PVDF) over the CNTs surface changes the surface characteristics of the electrode (previously confirmed with the Cdl values) and the mechanism of the DIC oxidation was changed from adsoption controled to diffusion controled process.

Figure 11 Linear fit of logi – logv dependence for a) PVDF/CNTs SPE modified electrode and b) CNT-SPE commercial electrode.
According to the previous results and regarding the CNTs electrodes as good electrochemical sensor for diclofenac chloride, we can considered and PVDF/CNTs electrodes as good and reliable sensing material for diclofenac detection. The morphologies of electrodes with different modifications were studied by SEM. The obtained images show that there are clear differences between the morphologies of the electrode’s surfaces. The formation of a polymer layer can be confirmed on Figure 12.a & 12.b for PVDF/CNTs modification, tested in acidic environment at pH 4.5 and these images show different morphology from commercial electrodes indicating an interaction between the surface and the acidic environment. It can be seen that acidic environment corrosively affects the electrode’s surface.
In the summary of this work, a simple, sensitive, time-saving and low cost procedure using an SPE PVDF/CNTs was presented and successfully applied for the determination of Diclofenac chloride (DIC). Physical characterization made with SEM and AFM confirmed the surface morphology and grain topography of the working electrodes as well as the roughness changes. The modified sensor showed an improved sensing activity towards DF compared to a bare SPE due to the effect of the polymer modifier. The use of modified CNTs improved the electron transfer process and the active surface area of the sensor. The SPE - PVDF/CNTs exhibited excellent current responses towards DIC determination and good linearity in the kinetic analysis. Furthermore, the SPE - PVDF/CNTs also showed satisfactory repeatability, reproducibility, and selectivity towards potential interferences which contribute to the benefits of this fast and low-cost method.
This work was financially supported by the bilateral scientific project between Faculty of Technology and Metallurgy – University Ss Cyril and Methodius in Skopje, Republic of North Macedonia and Technical University in Graz, Austria.
There is no conflicts of interest.

©2023 Grozdanov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.