eISSN: 2574-9927


Research Article Volume 7 Issue 4
Faculty of Engineering, Faculty of Chemistry UAQ, Autonomous University of Queretaro, Mexico
Correspondence: Robles Sanchez Ana Paola, Faculty of Engineering, Faculty of Chemistry UAQ, Autonomous University of Queretaro, Mexico
Received: December 13, 2023 | Published: December 27, 2023
Citation: Paola RSA, Contreras OEO, Hernandez AC. Effect of solvent and pH on the stability and chemical reactivity of the delphinidin molecule. Material Sci & Eng. 2023;7(4):223-226. DOI: 10.15406/mseij.2023.07.00227
The study of the stability in different solvents and its effect on the chemical reactivity for the delphinidin molecule is presented, using molecular modeling as a tool. The structural modeling of delphinidin in the different solvents (water, methanol, ethanol and acetone) was carried out based on the predominant structures of delphinidin in different pHs (1, 4.5, 5.5 and 7). Quantum mechanical studies were performed using density functional theory (DFT) with a B3LYP level and a base set 6-11+g(d,p). The solvation energies of the delphinidin molecule were obtained, finding that methanol is where the molecule dissolves best. Besides, The chemical reactivity indices for the molecule were obtained due to the effect of the solvent, finding that when it is found in water as a solvent, it acquires a lower hardness, making it more susceptible, that is, more reactive. The results also showed that at pH < 2 using methanol as solvent, the hydrogen atom of the hydroxyl group attached to the 4' carbon is the most prone to donate hydrogen.
Keywords: anthocyanins, delphinidin, solvent effect, pH, DFT
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
Anthocyanins belong to the family of flavonoids, as they are metabolites generated by the same biosynthetic pathway in plants1 and they confer blue, red or purple pigmentation to various flowers, fruits and vegetables,2 such as berries (blackberry, elderberry, grape, raspberry, etc.), cherry, strawberry, purple corn, yellow sweet potato, pomegranates, and red onion.3,4 Anthocyanins have recently been widely studied for their various pharmacological activities (antioxidant activity, prevention of neurodegenerative diseases, cardioprotection).5,6 Anthocyanidins are the aglycone form of anthocyanins, the latter having one or more sugars linked to the oxygen of C3 or C3 and C5 mainly (see Figure, for numbering). In addition, of the more than 600 anthocyanins that have been identified, only 5 of these are found in greater abundance and are classified according to the hydroxyl groups of the "flavylium" skeleton that characterizes the anthocyanidin.7,8
The pH plays a fundamental role in the structure of anthocyanins by promoting acid-basic, isomerization or addition reactions of a water molecule. For a pH less than 3, the main structure is the flavylium cation (HA+), which has a pKa from 4-5 for the formation of the conjugate base (quinoidal base). A nucleophilic addition at carbon 2 of H2O results in the formation of the colorless hemiacetal form, which upon an increase in pH leads to the formation of its colorless tautomer Chalcone (Figure 1).9,10 Apart from the importance in the pigment that it adopts when the pH varies, in its use as a natural colorant in food additives, in oenology11 or even as an intelligent indicator in the packaging of products to guess its freshness,12 also during digestion, the different pH levels in the body favor the most probable state of one structure or another.
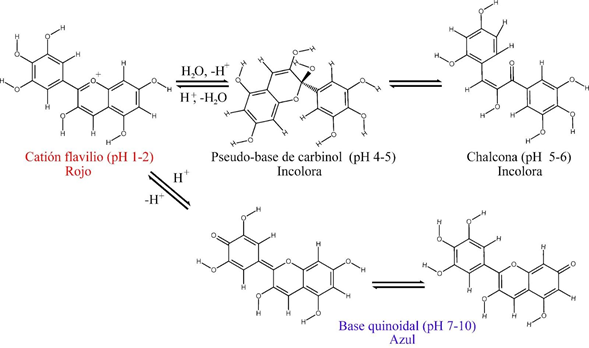
Figure 1 Delphinidin structures at different pHs. Flavylium cation (pH 1-2) shows the predominance of the oxonium ion in ring B. Pseudo-base of carbinol (pH 4-5) product of the hydration reaction of the flavylium cation. Quinoidal base (pH 7-10) product of the acid reaction of the flavylium cation, the conjugated pi bonds of the structure provide absorption in the visible spectrum. Chalcone (pH 5-6) tautomer of carbinol pseudo-base molecule, resulting from pyrilium ring opening, with H2Or as a catalyst. Structures reported in.6
Delphinidin together with cyanidin is the main anthocyanin present in blueberries, pomegranates, red grapes, among others,7 the first being the second most abundant in plants and vegetables, with only 12 % in proportion, therefore, it is necessary an arduous work of purification, where the solvent plays an important role.13 In this work, the stability of the delphinidin molecule with different solvents was analyzed using the different predominant structures according to the pH,14 using the continuous polarization model (SMD), in order to elucidate the mechanism by which they reach their most stable configuration, as well as their levels of chemical reactivity.
Optimization and frequency calculation
The DFT ab initio method was used for the optimization of four delphinidin geometries to obtain the geometry of minimum energy corresponding to the pH ranges: 1-2, 4-5, 5-6, and 7-10 in its ground state. (EF) considering the flavylium ion with a positive charge (+1), using the Density Functional Theory (DFT), the exchange correlation functional used was B3LYP (Becke, 3 parameters, Lee–Yang–Parr) [12 and the basis set with a fuzzy function “+” and polarization functions (p,d): 6-311+g(d,p). All the calculations were carried out with the Gaussian 09 software package. In addition, the frequency calculations were carried out, to obtain the thermodynamic parameters and which were taken as a reference and compared with what was reported in the literature: TD-DFT calculations were also carried out, an extension of the Density Functional Theory to treat excited systems and with this methodology the UV spectra were calculated, to determine electronic excited states reached in the different geometric structures proposed.15
Solvent effects
Four pure organic solvents were evaluated to calculate the stabilization or solvation energy of delphinidin in the medium, namely, acetone, water, methanol and ethanol. For each structure in the EF first without solvent effect and then the solvation effect was calculated, using the universal solvation model based on density (SMD), representing the solvent implicitly as a dielectric medium and its surface tension at the limit between the solute and solvent.13 Each solvation energy was calculated as the difference of the Gibbs free energy in its solvated state minus the Gibbs free energy in a vacuum. Additionally, chemical descriptors were obtained, such as hardness (the), the chemical potential (m), softness (p), electronegativity (x) and electrophilicity index (o)using the values of the HOMO and LUMO frontier orbitals. Where I=-HOMO and A=-LUMO, for the different geometries formed depending on the pH value. ecs. (1-5).
(1)
(2)
(3)
(4)
(5)
While the charges were evaluated as point charges centered on the atoms, derived from the electrostatic potential in specific CHELPG.
Delphinidin structure
The optimized structure obtained in the gas phase for the cationic isomer (Flavylium cation) is completely flat (Figure 2). dihedral angles ω1 (C2-C3-O-H), ω4 (C3′-C4′-O-H), ω5 (C2′-C3′-O-H), y ω6 (C4′-C5′-O-H) are anti-peri-planar, maximizing the distance between the hydrogens with partial positive charge of the hydroxyl group, while ω2 (C5-C6-O-H) and ω3 (C6-C7-O-H) are syn-peri-planar, which is in agreement with the literature,14,15 only with the difference in the angle ω3, which in this case is sin-peri-planar that can suggest that the following structure is a local minimun not global.
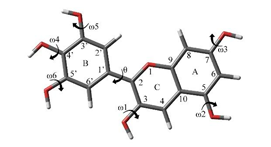
Figure 2 Optimized structure of delphinidin in the gaseous state.14
The structure with the lowest solvation energy obtained according to Eq. (6) corresponds to the cationic geometry (pH<2).
(6)
Where ΔG(solv) is the energy of solvatation, G(e.s) is the free Gibbs energy in the solvated state, and G(e.g) the free Gibbs energy in the gas phase.
According to the results that can be summarized in Figure 3, the stabilization of the different structures experimentally proposed in16 and calculated in this work, it can be seen that the oxonium ion of the flavylium structure (species formed at pH= 1), is the one that stabilizes the most in any solvent, with respect to molecular structures. This is consistent with the experimental part, where the pH selected to purify the compound is just at this pH. There are small differences in the stability of the molecule in the flavylium-type structure, being from higher to lower methanol > ethanol > water > acetone, the dielectric constants for these solvents are 0.791, 0.789, 0.998, 0.788, respectively. From here we can see that when the dielectric constant is in the range of 0.8 - 0.78 there is an increase in solubility as the polarity of the molecule increases in its different structures formed at different pH, the differences being more significant at pH=4. -5 and pH= 5-6. Unlike other solvents, acetone does not form hydrogen bonds, which is why a marked decrease in solvation energy is seen. On the other hand, between pH 4-5 and pH>7 there does not seem to be a significant difference, this may be due to the fact that both are neutral molecules, which is consistent with experimental results.17
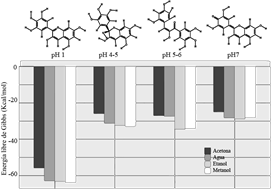
Figure 3 Graph of the solvation energy of the different structures pH < 2: Flavylium cation, pH 4-5: Pseudo carbinol base, pH 5-6 Chalcone and pH > 7 Quinoidal base evaluated in different organic solvents.
Chemical reactivity
HOMO and LUMO are known as the frontier orbitals and are the main ones involved in chemical reactivity, HOMO provides us with information about the ability to donate an electron while LUMO, the ability to accept an electron, the energies of these orbitals are used. to calculate the chemical reactivity descriptors. The first corresponding to hardness η, which represents the resistance to deformation or polarization of the electron cloud. According to Figure 4 (upper part), the carbinol pseudo-base is the one that presents the greatest stability, followed by Chalcone, that is, the two colorless compounds, while according to softness, the chromophores at pH 7 and pH 1 have the highest reactivity (Figure 4 & Tables 1–4).
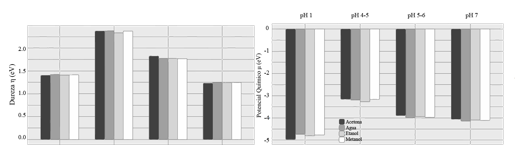
Figure 4 From top to bottom: Graph of hardnessthe and chemical potentialm of the different structures pH 1: Flavylium cation, pH 4-5: Pseudo carbinol base, pH 5-6 Chalcone and pH>7 Quinoidal base evaluated in different organic solvents.
|
|
DG(solution) |
m |
the |
p |
h |
oh |
|
Acetone |
-55.913 |
-4.969 |
1.402 |
0.357 |
4.969 |
8.804 |
|
Water |
-62.923 |
-4.741 |
1.422 |
0.352 |
4.741 |
7.904 |
|
Ethanol |
-63.243 |
-4.803 |
1.411 |
0.354 |
4.803 |
8.177 |
|
Methanol |
-63.974 |
-4.771 |
1.414 |
0.354 |
4.771 |
8.050 |
Table 1 Solvation energies (Kcal/mol), chemical potential, hardness, softness, electronegativity and electrophilicity index of the delphinidin molecule at pH 1
|
|
DG(solution) |
m |
the |
p |
h |
oh |
|
Acetone |
-26.004 |
-3.158 |
2.373 |
0.211 |
3.158 |
2.101 |
|
Water |
-31.233 |
-3.197 |
2.377 |
0.210 |
3.197 |
2.150 |
|
Ethanol |
-32.447 |
-3.270 |
2.334 |
0.214 |
3.270 |
2.291 |
|
Methanol |
-33.009 |
-3.166 |
2.374 |
0.211 |
3.166 |
2.111 |
Table 2 Solvation energies (Kcal/mol), chemical potential, hardness, softness, electronegativity and electrophilicity index of the delphinidin molecule at pH 4-5
|
|
DG(solution) |
m |
the |
p |
h |
oh |
|
Acetone |
-24.559 |
-3.849 |
1.805 |
0.277 |
3.849 |
4.103 |
|
Water |
-29.594 |
-4.002 |
1.755 |
0.285 |
4.002 |
4.563 |
|
Ethanol |
-30.414 |
-3.932 |
1.752 |
0.285 |
3.932 |
4.411 |
|
Methanol |
-30.247 |
-3.967 |
1.746 |
0.286 |
3.967 |
4.505 |
Table 3 Solvation energies (Kcal/mol), chemical potential, hardness, softness, electronegativity and electrophilicity index of the delphinidin molecule at pH 5-6
|
|
DG(solution) |
m |
the |
p |
h |
oh |
|
Acetone |
-25.031 |
-4.057 |
1.232 |
0.406 |
4.057 |
6.680 |
|
Water |
-28.352 |
-4.146 |
1.251 |
0.400 |
4.146 |
6.868 |
|
Ethanol |
-28.993 |
-4.088 |
1.242 |
0.403 |
4.088 |
6.728 |
|
Methanol |
-28.162 |
-4.115 |
1.246 |
0.401 |
4.115 |
6.796 |
Table 4 Solvation energies (Kcal/mol), chemical potential, hardness, softness, electronegativity and electrophilicity index of the delphinidin molecule at pH 7
Molecule charge
In Figure 5, the partial atomic charges calculated with CHELPG are shown, for the pure gaseous state of the solute and with the solvents water and methanol. In the pH<2 structure, it can be seen that the oxonium ion of the flavylium structure, O1 has a lower negative charge than the other oxygens, in gaseous form (-0.291), it does not reach a +1 charge on this atom due to the charges are distributed over all the atoms in the molecule.
however, compared to the remaining oxygen atoms in the molecule, O1 is slightly more positive. The second oxygen with the highest partial positive charge is 4'C-OH, therefore, acting as a weak acid (AH), it could be a site for the elimination of radicals by the mechanism of hydrogen transfer to the reactive species (R·) (HAT), as shown in the following reaction:
R + AH→ RH + A·
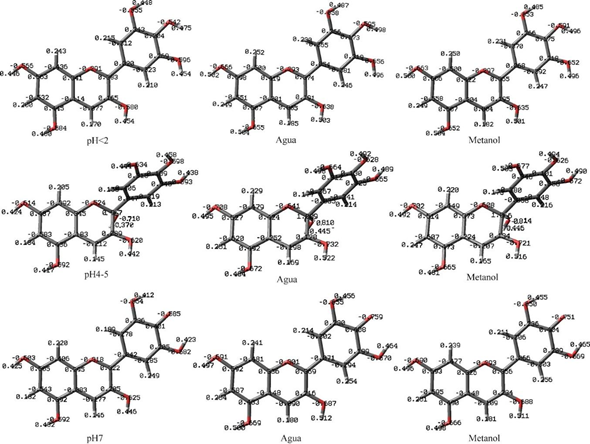
Figure 5 Partial atomic charge calculated with CHELPG, for the pure gaseous state of the solute and with the solvents water and methanol.
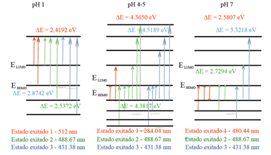
Figure 6 Excited states and electronic transitions of the predominant delphinidin molecule at 3 different pH levels.
The conjugate base product of this reaction (quinoidal base) is stabilized by hydrogen bonds of the 3'C-OH and 5'C-OH hydroxyls.,14 after this, the O of 5C-OH is the most positive, so at pH 7, it could be a possible acid group, this being an important structure in the oral cavity or in the human intestine, varying the pH in a range of 5.6-7.9 and 6.7-7-4 respectively.17
Calculation of excited states TD-DFT
In Fig. 6, the excited states obtained with the TD-DFT calculation are shown. According to the diagrams shown in Fig. 6 that were obtained with the TD-DFT calculation, the electronic excitations go from HOMO, HOMO-1, HOMO-2 to LUMO, LUMO+1, LUMO+2, LUMO+ 3, mainly. These excitations correspond to the transitions of the orbitals: n → π*, π → π* y π → σ*.
Excitations with wavelengths between 400 nm and 600 nm are what give color to molecular systems. The ΔE of the energy gap, ΔE= |EHOMO -ANDLUMO|, calculated for each system, turned out to be lower for the oxonium ion of the flavylium structure formed at pH=1 than for the other compounds, thus confirming that this compound with this characteristic turns out to be the most prone or sensitive to the effect of half.
Using the density functional theory as a useful tool in the description of the structural and electronic properties of molecules, it was possible to know the solvation and stabilization energy of the delphinidin molecule, where it was found to have greater stability. in the different solvents used experimentally when it acquires an oxonium ion structure or also known as flavylium, which is formed at pH=1.0. In addition, the partial atomic charges of the molecule were calculated to predict the group with the greatest antioxidant potential, among the solvents used, however, in the sources of consumption it is found in the glycosylated form, which has reported a decrease in antioxidant activity, in addition to the fact that it undergoes a breakdown during digestion where one of its predominant metabolites is protocatechuic acid and caffeic acid, so its antioxidant effect and therefore its therapeutic effects can also be considered to these metabolites.183 According to the results obtained through this type of study, it is possible to better understand the chemical nature that a molecular system can have and the effect that the medium (solvent) can cause from the electronic point of view.
We expressed our gratitude to the Autonomous University of Querétaro, the Autonomous University of Mexico Iztapalapa campus: Parallel Supercomputing and Visualization Laboratory (LSVP) of the UAM-Iztapalapa Unit for the computing resources used.
There is no conflict of interest.

©2023 Paola, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.