eISSN: 2574-9927


Mini Review Volume 3 Issue 2
Dongguk University, South Korea
Correspondence: Huseynova G, Dongguk University, Pildongro 30, Jung-gu, Seoul, South Korea
Received: December 26, 2018 | Published: March 29, 2019
Citation: Huseynov G. Cationic species as dopants for organic semiconductors. Material Sci & Eng. 2019;3(2):51-53. DOI: 10.15406/mseij.2019.03.00089
Herein a short review of cationic species reported as dopants for organic semiconductors is presented. The approach suggests using the same cationic species for both p- and n-type doping of organic matrices with organic dopants. As an example, pyronin B chloride and benzyl viologen dichloride are reviewed as universal dopants working both ways. Different device characteristics and measurements confirm the donor and acceptor properties of these organic molecules.
Keywords: doping, organic semiconductors, n-type dopant, p-type dopant, benzyl viologen dichloride, organic field-effect transistors, conjugated polymers
Organic semiconductors (OSCs) are the potential key materials for future flexible electronics due to their outstanding mechanical and optoelectronic properties1,2 Electronic devices such as organic light emitting diodes have already shown successful progress for flat panel displays, and application of OSCs is gradually expanding to various fields of electronics.1–3 However, OSCs still lag behind inorganic ones due to their poor electrical properties including low charge carrier mobility and conductivity as well as device stability issues.4–7 In order to overcome these issues, several approaches have been developed one of which is doping.5 Doping is one of the most effective methods to improve electrical properties of OSCs through increasing their charge carrier density and mobility.2,8–11 However, the doping of OSCs is different from the doping of inorganic ones. Unlike the latter, doping in organic electronics does not assume the replacement of a host lattice atom by an impurity atom. It is rather a simple charge transfer between two molecules.2,8,9,12–14 A significant number of research groups have reported different kinds of dopants for OSCs. In this review, a summary of the dopants, namely cationic species that can be applied as both p- and n-type dopants, is introduced.
Pyronin B
As the first example, pyronin B dichloride (PyB) is given. It is a solution processed cationic non-polymeric dye. PyB belongs to xanthene dyes, which are salts of organic bases. They are called cationic dyes, because their molecules ionize in solution, causing the colored component to become a positively charged radical which reacts with the anionic sites of the other molecules they are blended with. Accordingly, they act as an electron deficient site, which makes them perfect p-type dopants. PyB was reported as a p-dopant for diketopyrrolopyrrole-thieno [3,2-b]thiophene (DPPT-TT), an ambipolar conjugated copolymer, in a simple solution-processed doping.15 The doped polymer was used as an active layer for OFETs. As a result, device performance including hole mobility and threshold voltage was significantly improved (Figure 1). Pyronin B was also reported as an effective and stable n-type dopant when reduced to its neutral leuco (colorless) state through both solid-state ad solution-processed doping.16–18
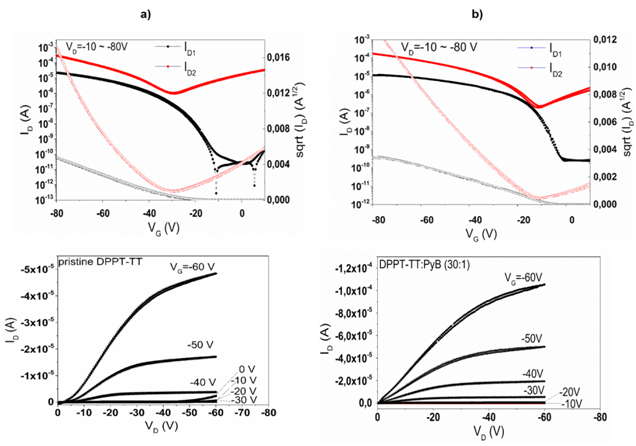
Figure 1 Representative transfer and output curves for DPPT-TT OFETs: (A) un doped; (B) PyB doped (30:1). ID, VD, VG – drain current, drain voltage and gate voltage, respectively.
Viologens
Another cationic compound, benzyl viologen dichloride (BVD) was recently reported as an effective n-type dopant for OSCs when reduced to its neutral state. BVD consists of two chlorine anions and a positively charged organic molecule with two nitrogen cations. BVD was used as the n-type dopant, since it exhibits strong electron donating properties originating from the viologen unit due to low reduction potentials and redox-rich nature. All viologen compounds, including benzyl viologen, typically undergo three reduction states in redox reactions, forming different colored compounds in solution. Their initial energetically stable cation (V2+) is colorless, whereas the single electron reduced form (radical mono cation, V+) is intensely violet, and the third reduced state (neutral V0) is yellow. The last two states both readily transfer their electrons but the neutral state is the most reactive, and a stronger reducing agent than the mono cations due to the lower reduction potential. Benzyl viologen has the lowest reduction potential among all viologens. Benzyl viologen reduction potentials are in the range from -0.33 to -0.79 V for the V2+, and V0 and V+ states, respectively. To use BVD for doping purposes, it was reduced chemically to obtain the electron donating neutral state from the di-cation form. As a result, the polymers doped with BVD exhibited high n-channel performance when applied to OFETs (Figure 2).
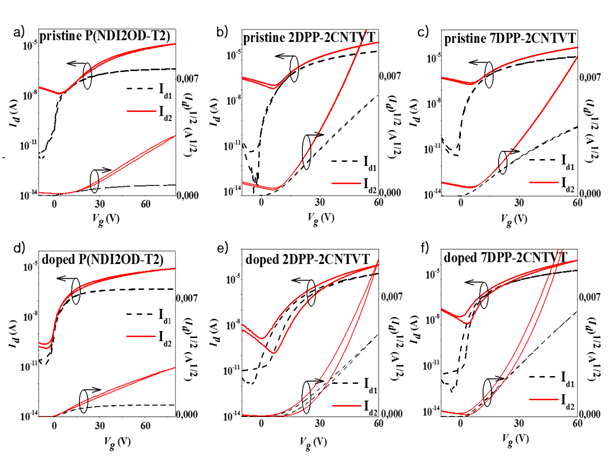
Figure 2 Transfer characteristics for pristine and doped (A) and (D) P(NDI2OD-T2), (B) and (E) 2DPP-2CNTVT, and (C) and (F) 7DPP-2CNTVT OFETs, respectively. Id1 and Id2 are the drain currents in linear (Vd=10V) and saturation regimes (Vd=60 and 80V for 2DPP-2CNTVT and 7DPP-2CNTVT, and P(NDI2OD-T2) based devices), respectively. Id, Vd, and Vg stand for the drain current, drain voltage, and gate voltage, respectively.
This article reviewed n- and p-type doping effects of two cationic compounds, PyB and BVD for various organic semiconductors. These two examples of cationic species have demonstrated that the same cationic compound can be effectively used as both p-type dopant when used as is and n-type dopant when reduced to their neutral states. The current study suggests a new class of universal dopants for organic electronics and will inspire follow-up approaches in this field.
Author declares that there is no conflicts of interest.

©2019 Huseynov. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.