eISSN: 2574-9927


Review Article Volume 2 Issue 1
1Department of Earth Sciences, Adekunle Ajasin University, Nigeria
2Department of Geology, University of Ibadan, Nigeria
3Hawaii Institute of Geophysics and Planetology, USA
Correspondence: Cyril C Okpoli, Department of Earth Sciences, Adekunle Ajasin University, Nigeria
Received: November 24, 2017 | Published: January 29, 2018
Citation: Okpoli CC, Oladunjoye MA, Bervera EH. A review on rock magnetism. Material Sci & Eng Int J. 2018;2(1):15-17. DOI: 10.15406/mseij.2018.02.00026
Crustal rocks have a magnetic memory that may endure for millions or even billions of years due to composition of iron oxides. The secret behind that permanence lies in the high temperatures to which the minerals were exposed-near their Curie temperatures of several hundred degrees Celsius- while cooling in Earth’s magnetic field and in the stabilizing influence of the cooling process itself. This memory is known as thermal remanent magnetization (TRM). It is much more resistant to subsequent fields than the more conversant remanent magnetization due to fields applied at ambient temperatures in a computer’s hard drive, for example. Still, a small fraction of the TRM responds to and records Earth’s field during later heating events, such as the burial of the rocks during mountain building/ plate subduction.
Keywords: rock magnetism, magnetic memory, iron oxides, temperature
Rock magnetism is the study of rock properties of rocks and its magnetic minerals. A very significant issue in rock magnetic studies relates to the deduction on the magnetic remanence carrier, and how the rocks became magnetized. We review present knowledge on the importance of natural magnetic phases, how to identify them, how they were formed, and what their magnetic behavior is.1,2
Iron is by far the most abundant transition element in the solar system. Largely, rock magnetic studies rely on the magnetic iron-bearing minerals: the iron-nickels (principally important for extraterrestrial magnetic studies); the iron-oxides: magnetite, maghemite, and hematite; the iron-oxyhydroxides goethite and ferrihydrite; and the iron-sulfides: greigite and pyrrhotite.
Rock magnetic studies of host rocks and ore minerals produce information on the concentration, composition, and microstructure of the magnetic mineral fraction. This is important because of quantitative interpretation of detected magneto metric anomalies. This information is also needed for the investigation of problems related to the magnetic history of rocks and anomalies in the geomagnetic field, and to problems in geophysics and geochemistry. Data on rock magnetic properties can restrict the range of possible models, particularly when magnetic properties are methodically obtained as part of complementary data from geological boreholes and other geophysical information. Thus, rock magnetic parameters are crucial for a better understanding of the relationships of geology and rock magnetism and magnetic anomalies. This relation is complex because of the need of magnetic signature with respect to a rock unit, structure, lithology and geologic history.
Great advances in data acquisition, processing, satellite navigation, and image processing of high-resolution aeromagnetic surveys, resulted in a critical need for a better appreciation of these relationships. Aeromagnetic surveys supply information on sources at all depths within the crust: they have been widely used for decades in mineral and petroleum exploration. Software now allows for advanced modeling. Magnetic interpretation has the potential for modeling rock bodies; however, a major restrictive factor has been the non-uniqueness of solutions of models. The two most important constraints on magnetic models are reliability of magnetic properties and of petrophysical data, and the known geological structure. Without a solid constraint, the non-uniqueness in potential field data limits the amount of information and knowledge we can retrieve from magnetic surveys. Given the amount of money spent on acquisition of magnetic data, and the use of magnetic surveys in exploration, detailed studies of rock-magnetic properties will strongly be needed to support modeling of magnetic anomalies.
At present, it is recognized that rock magnetic information assists in optimizing magnetic anomaly interpretations, especially for magnetite-bearing ores and related rocks. The intensity of remanent magnetism varies greatly in rocks and Fe-ores, and it may exceed the intensity of induced magnetization implying high Q-ratio values.3 Sometimes, scattered paleomagnetic directions from exposed outcrops suggest the effect of lightning strikes and imply extremely high values of magnetization. Several authors have focused on different aspects, e.g., relationships between petrology, observed aeromagnetic anomalies and physical properties to define geological and structural units and the mapping of these units to establish a correspondence between magnetic petrology and rock-magnetic properties.
Basically, all materials are magnetic since they have electrons that have magnetic moments and electron’s motion results in currents with their associated magnetic fields. Magnetism is often subdivided into: induced magnetization and permanent magnetization. Induced magnetization is described by the equation
where M is the magnetization, H is the magnetic field, and is a second order tensor denoted as the susceptibility. For example, diamagnetic material like halite, NaCl have negative while , paramagnetic like iron-rich olivine or fayalite, Fe2SiO4 have positive .
Earth scientists attribute permanent magnetism to exchange energy and not to soul. A permanent magnetic command results from exchange energy, which combines Pauli Exclusion Principle and Coulomb interaction energy. So, exchange energy can only accurately be understood through quantum mechanics. Exchange energy is often designed by evaluating the nature of electron orbitals overlap. Reliant on the specifics of the electron overlap, a minimum in exchange energy often requires that adjacent atoms have parallel magnetic moments and the substance is known as ferromagnetic. With different overlap, the minimum in energy occurs when the adjacent magnetic moments are antiparallel. Then the material is denoted as antiferromagnetic when the moments are of equal magnitude and ferrimagnetic when the adjacent magnetic moments vary in magnitude. The best-known example of a ferrimagnetic mineral is magnetite). Magnetite (Fe3O4) is an example of ferrimagnetic mineral and it have an indirect exchange energy because the coupling of adjacent iron atoms arises through intervening oxygen atoms.
The increase in temperature of a substance of thermal energy (quantized lattice vibrations known as phonons) increases and ultimately overcomes the exchange energy. The peak temperature a ferromagnetic material can possess a magnetic structure in the absence of an external magnetic field is known as Curie temperature. Néel temperature is the permanent loss of magnetic order in antiferromagnetic and ferrimagnetic structures. (Some Paleomagnetists also call it- the Curie temperature.) The saturation magnetization monotonically increases on cooling from the Néel/Curie temperature to absolute zero providing no phase transformation occurs on cooling. The Curie temperature at ambient pressure of iron, a ferromagnetic substance, is 1043K and the Néel temperature of ferrimagnetic magnetite is 853K. For instance, Ilmenite (FeTiO3) is an antiferromagnetic material and it has a Néel temperature of 40K. It is advantageous to recognize that the melting temperature of a magnetic substance always exceeds the Curie/Néel temperature. (So-called magnetic fluids contain suspended solid magnetic material.) Because the electron overlap is a function of pressure, the Curie and Néel temperatures are also functions of pressure.4
As rock magnetic studies illustrate during heating and cooling cycles substantial changes happen in magnetic properties. These transformations may involve the formation of new magnetic substances, change of Curie point, increase or decrease of magnetization, etc. Many studies show changes in rock magnetization during heating at temperatures in the range of 200° to 500°C.5
Most times, the samples exhibit bimodal blocking-temperature spectra. Such bimodal blocking-temperature spectra with blocking temperatures in the range of 200-580°C were reported in rocks from different regions.6 Some results establish transformations in both the value of TC and the quantity of magnetic minerals within the rock during tests of heating-cooling cycles at temperatures in the range of 350-580°C.7 Some authors find a temperature range rather than at an exact value of blocking temperatures.8 Several researchers believe that a special role in rock magnetism belongs to maghemite ( -Fe2O3) and/ or titanomaghemite.9 However, even though maghemite has very strong magnetic properties, it is metastable and would be effortlessly transformed to antiferromagnetic hematite. The temperature range of the stability of maghemite is usually within 200-350°C.10
Large differences in Curie temperature (Tc) and blocking temperature could be related to iron-oxide changes and re-magnetization progressions. Processes of heating, cooling, reheating, and further cooling could take place in different regions and during different processes-recurrent intrusions or extrusions, hydrothermal events, recurrent metamorphic processes. Such courses can change both the magnetic phase and content in the rocks of a section. Even though magnetite has a TC between 575°C and 580°C, this does not mean that all rocks containing magnetite formed above the TC and acquired magnetization at this TC during cooling. Magnetite could be formed by iron-oxide transformations at any temperature within the range of iron-oxide transformations (between 570°C and 200°C), hitherto the newly produced magnetite would still have the same properties and TC as would a magnetite formed under varied temperature conditions. Evidently, magnetic minerals cannot gain a remanent magnetization before formation of the magnetic phase. Moreso, a magnetic phase formed above its TC cannot acquire magnetization until the temperature is reduced to its blocking temperature. TMs, formed at high temperature, cannot get magnetization till it cools to the blocking temperature. Quick cooling can prevent rocks from substantial iron-oxide transformations. Thus, the amount of magnetite would hinge on the cooling rate, unlike for rocks formed under varied conditions.
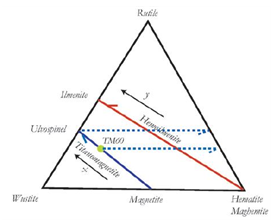
Composition in minerals is normally plotted on ternary diagrams such as shown in Figure 1. The oxides for these species are FeO (wüstite), Fe2O3 (hematite or maghemite, depending on structure), and TiO (rutile). Every point on the triangle represents action mixture or a solution that adds up to one action. Iron-oxide transformations might take place during either cooling or heating processes. They would start when the temperature within the cooling rock decreases below the upper boundary of the temperature range of iron-oxide fluctuations, and they would continue until the temperature drops below that of the lower boundary of the temperature range of iron-oxide variations. Iron-oxide fluctuations would also take place during the heating of a rock as soon as its temperature rises above the lower boundary of the temperature range of iron-oxide changes. Iron-oxide fluctuations s would start just above 200°C and transform the magnetic properties of the rock. This is constant with the well-known presence of broad temperature tails above and below the blocking temperature within which transformations in the composition of a magnetic phase and its magnetic characteristics take place.11 This may mean that the magnetic phase of a rock was either formed close to the blocking temperature, or that the blocking temperature does not control iron-oxide transformations.
None.
Authors declare there is no conflict of interest in publishing the article.

©2018 Okpoli, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.