eISSN: 2574-9927


Research Article Volume 1 Issue 4
1Industrial University of Santander, Colombia
2The University of Wolverhampton, UK
Correspondence: Carlos A Rios, Industrial University of Santander, Colombia, Fax 01902 322714
Received: June 17, 2017 | Published: November 30, 2017
Citation: Ríos CA, Williams CD, Fullen MA. Heavy metal removal using alkali activated kaolinite in the Cao-Al2O3 -Sio2 -H2O system. Material Sci & Eng Int J. 2017;1(4):116-122. DOI: 10.15406/mseij.2017.01.00019
The transformation of kaolinite was examined at 175°C for 24 h in the CaO-Al2O3-SiO2-H2O (CASH) system, which is important in cement science and especially in, cement chemistry and is closely related to the pozzolanic reaction, the CaO-aggregate reaction and the glass fibre reinforcement of hardened cement. The hydration products were characterized by X-ray diffraction, scanning electron microscopy, Fourier transformed infrared spectroscopy, Magic Angle Spinning Nuclear Magnetic Resonance and thermogravimetric analysis in order to elucidate their mineral chemistry and microstructure. Results reveal that several poorly crystalline phases were formed, with un-reacted Ca(OH)2 appearing at shorter reaction times. Hydrogarnet tends to form more rapidly than tobermorite. It was transformed into aluminium-substituted tobermorite with curing time. A batch experimental study confirmed that kaolinite-based calcium silicate hydrates are effective for the treatment of acid mine drainage, particularly in removing metal ions and ammonium.
Keywords: kaolinite, cash system, cement, calcium silicate hydrates, hydrogarnet, tobermorite
Most of calcium silicate hydrate or calcium aluminosilicate hydrate, CS(A)H, and related phases are relevant to Cement industry and have potential as geothermal well sealants as well as autoclaved construction materials. The CS(A)H system is highly complex and is comprised of crystalline to amorphous phases with variable composition that build up a wide family of phases, which are interesting for the variety of their structural arrangements, the peculiarity of the transformation processes in which they are involved and the relationships with compounds which form during the hydration of the Portland cement.1 Different studies have been carried out on the hydrothermal transformation of kaolinite or metakaolinite into CS(A)H phases, with particular attention on tobermorite. The most widely studied aluminium-bearing materials and their effects on various aspects of tobermorite formation include: g-Al2O3, Al(OH)3, kaolinite, metakaolinite, zeolite and solid waste materials. The raw materials used in the synthesis of tobermorite usually contain aluminium-bearing impurities and therefore the role of aluminium in the stabilization of tobermorite has been reported in several studies.2–10 A chronology of the main results from these studies is presented as follows.11,12 undertook a microstructural study on autoclaved clinker and slag-lime pastes in the presence and absence of silica sand using scanning electron microscopy, determining the formation of several hydration products, such as C-S-H, hydrogarnet and tobermorite. The formation of hydrogarnet for ratios of Al/(Si +Al) in the range of 0.12–0.50 when kaolinite is used as the source of aluminium was reported by.13 Mitsuda3 studied the effect of substitution of Si by Al in tobermorite formed in the CaO-SiO2-H2O system. Van Aardt14 investigated the reaction between Ca(OH)2 and minerals containing alumina, which forms C4A-hydrates and/or hydrogarnet (calcium-alumina-silicate hydrate), depending on temperature. Serry et al.15 studied metakaolin-lime hydration products, determining that gehlenite hydrate (C2ASH8) was the main hydration product and its amount increased with reaction time, whereas hydrogarnet crystallized at the early stages of hydration in the low-lime content mixtures, increasing with curing time. Atkins et al.16 studied the solubility properties of ternary and quaternary compounds in the CaO-Al2O3-SO3-H2O system, with ettringite (C6A3H32) and hydrogarnet (C3AH6) dissolving congruently, whereas monosulphate (C4AH2) and tetra-calcium aluminate hydrate (C4AH13) dissolved incongruently. The reaction mechanism of the hydrothermally treated CaO-SiO2-Al2O3 and CaO-SiO2-Al2O3-CaSO4 systems was conducted by Al-Wakeel & El-Korashy,17 using mixtures of CaO, amorphous SiO2 and kaolinite in the presence or absence of SO42– ions (added as CaSO4·2H2O), which were treated in suspension under hydrothermal conditions at temperatures of 80-200°C. The results indicate the occurrence of several C-S-H phases, which are then transformed into Al-substituted 11Å tobermorite. In both systems excess Al2O3 appeared as a hydrogarnet phase.18 First reported the occurrence of hydrogarnet-type cubic crystals in polymer-modified mortars.19 investigated the hydrothermal conversion of kaolinite with calcium hydroxide and dissolution of reaction products in hydrochloric acid, obtaining different reaction products, such as hibschite, omisteinbergite, tobermorite and jaffeite. Klimesch and Ray investigated the effects of quartz particle size on hydrogarnet formation and the nature of hydration products in unstirred autoclaved metakaolin-lime-quartz20–22 and kaolin-lime-quartz23 slurries. Hydrogarnet was always among the first phases formed and invariably appeared before 11Å tobermorite and with increasing reaction time the amount of hydrogarnet decreased and finally disappeared, while Al-substituted 11Å tobermorite increased concurrently, and the continued existence of hydrogarnet depended on such factors as reaction time and initial Al2O3 content in the raw mixture.23 Klimesch et al.22 monitored the evolution of hydration products in unstirred autoclaved metakaolin–lime–quartz slurries with reaction time using thermogravimetric analysis, concluding that hydrogarnet was always one of the first phases formed at all metakaolin additions and invariably appeared before tobermorite. Frías et al.24 studied the effect of curing temperature on the reaction kinetics of a metakaolin/lime mixture, determining from thermal analysis (TG and DTA) data different appearance sequence of the hydrated phases. Coleman et al.25 investigated the synthesis of hydrogarnet and tobermorite from newsprint recycling residue. A batch sorption study confirmed that the as-synthesized tobermorite was effective in removing Cd2+, Pb2+ and Zn2+ from acid aqueous media. Frías et al.26 evaluated the effect of different parameters on the reaction kinetics in metakaolin/lime and metakaolin-blended cement matrices at 60°C, defining different sequences of formation of the hydrated phases according to the matrix. Siauciunas et al.27 studied the influence of SiO2 modification on the formation and stability of hydrogarnet and Al-substituted 11Å tobermorite during hydrothermal synthesis, determining that hydrogarnet always tended to form more rapidly than tobermorite, although with increasing reaction time hydrogarnet started to fracture and its amount decreased. Frías et al.28 investigated the influence of metastable hydrated phases like C-S-H, C2ASH8 and C4AH13 on the pore size distribution and degree of hydration of metakaolin-blended cements cured at 60°C. According to them, these phases are stable at room temperature, but some (C2ASH8 and C4AH13) are metastable phases, converting to hydrogarnet for long curing times at elevated temperatures.29 Conducted a selective synthesis of phillipsite and hydrogarnet, employing Ca(OH)2 under mild chemical conditions, provided a potential alternative for the recycling of fly ash and investigated the heavy metal cation removal abilities towards lead ions of the synthetic phillipsite.30 Carried out XAS experiments using synchrotron radiation and concluded that the hydration of C4AF forms hydrogarnet in which Fe randomly substitutes for Al as well as an amorphous FeOOH phase, intermediate products like AFm (ettringite) are also formed but rapidly evolve to hydrogarnet, iron does not seem to be incorporated in the AFm structure. Therefore, the efficiency of the C-S-H materials in removing heavy metals should be considered.31 studied phase and microstructure evolution during hydrothermal solidification of clay-quartz mixture using marble as a lime source, with tobermorite and hydrogarnet being the major phases formed after reaction, which are responsible for strength development in the as-synthesized materials suitable for building.32 studied the effect of curing temperature on reaction kinetics in a metakaolin/lime mixture cured at 60°C and after 60 months of hydration, evaluating the stabilities of hydrated phases formed during the pozzolanic reaction. The results obtained by this author show that metastable hexagonal phases (C2ASH8 and probably C4AH13) coexist with a stable cubic phase (hydrogarnet) in the absence of lime.33 Carried out a study on solid-state 29Si and27Al NMR on Si-substituted hydrogarnet. The appropriate combination of NMR and Rietveld analyses of the X-ray and neutron diffraction patterns allowed better understanding of the formation of Ca3Al2(SiO4)3-x(OH)4x hydrates during the hydration of Ca3Al2O6 in the presence of nanospheres of amorphous silica.34 used 29Si NMR and 27Al MAS NMR spectroscopy to study autoclaved cement-quartz pastes containing 0-30.5% metakaolin as either quartz or cement replacement.
These aspects are very important from the point of view of the engineering and microstructural properties, which could have associated a negative effect on the performance of the as-synthesized products. The technological interest of tobermorite arose from its role as the primary binder of most autoclaved calcium silicate based building materials. However, more recently the ion exchange characteristics of synthetic 11Å tobermorite and its aluminium-substituted analogue have been investigated with respect to their potential in nuclear and hazardous wastewater conditioning.35–40
The objective of the study is to investigate the chemical transformation of kaolinite under hydrothermal conditions at 175°C for 24h and establish the reaction sequence and nature of the C-S-H phases formed. In addition, this study examines at laboratory-scale the effectiveness of kaolinite-based calcium silicates as sorbents in removing metal ions and ammonium from acid mine drainage (AMD), which could be used as environmentally friendly materials with high remediation abilities.
Materials
In the present study, kaolinite (Supreme Powder, ECC International, UK) or metakaolinite were used as starting materials. Kaolinite (composition 46.44% SiO2, 38.80% Al2O3, 0.03% TiO2, 0.52% Fe2O3, 0.08% MgO, 0.33% Na2O and 0.69% K2O) was used without further purification. Metakaolinite has extraordinarily high pozzolan reactivity and it was prepared by calcining kaolinite at 600°C in air for 2h. All solid starting materials were first ground and particles <75mm selected by sieving, before gels were prepared. Activator reagents included CaCO3 (Griffic), Al(OH)3 (Fisher Scientific International Company), precipitated SiO2 (BDH), and distilled water using standard purification methods. A sample of acid mine drainage collected from the abandoned copper-lead-zinc deposit at Parys Mountain (North Wales) was used as a natural solution in a batch experiment.
Synthesis of C-S-H phases
The method used in this study was based on that of Kalousek.13 The starting material was the following mixture: CaO+kaolinite, identified as KAOAds. Starting mixtures were prepared with molar compositions of Al/(Si Al) and Ca/(Si Al)=0.10-0.13 and 6.50-1.00. To activate the raw materials with calcium oxide solutions to synthesize hydrated calcium silicates, reagent-grade calcium carbonate (CaCO3) was calcined at 1000°C for 1h to obtain CaO. For comparison, a reaction gel was prepared using the following pure chemical reagents: amorphous Al2O3 (prepared by calcination of Al(OH)3 at 550°C for 1h), precipitated SiO2, CaO and distilled water. The resulting mixtures were transferred to 20cm3 PTFE-lined stainless steel autoclaves and the hydrothermal synthesis was performed under static conditions using water/solid ratios of 1.50-2.28 at 175°C for different curing times. On removal from the oven they were quenched in cold water and the product recovered by vacuum filtration using a filter funnel and Whatman paper to remove excess water. Once the water was extracted from the filtered product, the sample was rinsed with ethanol (C2H5OH) and dried at 80°C overnight. Further details of the gel compositions and synthesis conditions.
Characterization of calcium silicate hydrates
The synthesis phase(s) in the solids products were identified by powder X-ray diffraction (XRD), performed on a Philips PW1710 diffractometer equipped with Cu-Ka radiation (40kV and 40mA) and theta compensating divergence slits. Data were collected in 45min over the 2q range 3-50°, with a step size of 0.02° with phase identification being made by searching the ICDD powder diffraction file database. Surface morphology was investigated using a Zeiss EVO 50 scanning electron microscope equipped with secondary detector and EDX on gold-coated samples. Fourier transform infrared (FT-IR) spectra were recorded in the range 400-4000cm–1 on a Mattson Genesis II FT-IR spectrometer. However, we discuss only the 1200-400cm–1 region, because this is where the spectra show remarkable changes. MAS NMR spectra for 29Si and 27Al, respectively, were recorded at room temperature on a Varian Unity Inova spectrometer under the following analytical conditions: MAS probe 7.5 and 4.0mm; frequency 59.6 and 78.1MHz; spectral width 29996.3 and 100000.0Hz; acquisition time 30 and 10µs; recycle time 120 and 0.5sec; number of repetitions 15 and 2200; spinning rate 5040 and 14000Hz; pulse angle (DP) 90.0 and 18.9o. The chemical shifts were referenced to tetramethylsilane (TMS) for 29Si and 1M AlCl3 aqueous solution for 27Al. The 29Si chemical shift of the peaks were analysed using the Qn(mAl) classification, with the Si tetrahedron is connected to mAl and (n-m) Si tetrahedral where n=0 to 4 and m=0 to n. Thermogravimetry was performed on a Mettler Toledo TG50 thermobalance in the temperature range of 25-700°C, with a heating rate of 10°C min–1 under flowing air. Mass losses were determined by employing both TGA and DTG curves. The second derivative differential thermal curve was used for peak temperature determinations.
Batch experiment and water analyses
In order to investigate the efficiency of selected kaolinite-based calcium silicate hydrates for removing metal ions and ammonium from AMD, a laboratory batch experiment was conducted at room temperature. A weighed amount of sorbent (0.25g) was introduced in PVC plastic bottles of 100ml, then a volume of 20ml of AMD with pH=1.96 was added. Later, the sorbents and AMD were mixed by continuous shaking during 30minutes. At the scheduled reaction time the bottles were removed from the shaker and the adsorbents were separated by filtration, while filtrates were stored at 4°C in a refrigerator for chemical analyses. All measurements were performed according to the Standard Methods for the Examination of Water and Wastewater. The pH and electrical conductivity of the raw AMD and the filtrates obtained after batch experiments were measured using a pH 211 Auto-calibration bench pH/mV meter (Hanna instruments) and a Cond 315i conductivity meter (WTW instruments), respectively. The metal concentrations were determined using a Spectro Ciros ICP-AE Spectrometer. A Photometer 7100 fully integrated with the Palintest water test system was used to measure ammonia over the ranges 0-1.0mg/l N. Dilutions were made using distilled water, depending on the original EC of each sample.
One of the most interesting results observed during the preparation of lime mixtures was the exothermic reaction of CaO to form Ca(OH)2 immediately after the addition of the raw materials to distilled water, which was detected by a warming of the beaker containing the reaction mixture. As follows, we discuss the results corresponding to the characterization of the prepared mixtures by different analytical techniques and their application in the treatment of AMD through an adsorption batch experiment.
X-ray diffraction analysis
X-ray patterns and assignments of the peaks of original kaolinite and metakaolinite and their synthesis products are presented in (Figure 1). It is evident that forming reactions of calcium silicate hydrates started very rapidly, after hydrothermal reaction of the starting materials in the system CaO-SiO2-Al2O3-H2O. Figure 1A shows that the characteristic reflection peaks of kaolinite (at 12.34° and 24.64° 2θ) decreased with reaction time. Ca(OH)2 and C-S-H were the main phases identified at 0 h, along with relicts of kaolinite. The amount of Ca(OH)2 increased with curing time and reached a maximum after 1h. Hydrogarnet and C-S-H were the reaction products formed after 1.5h of treatment, with the maximum and minimum amounts of hydrogarnet and C-S-H, respectively, after 24h. Hydrogarnet did not disappear after 24h of treatment, and the formation of tobermorite was observed after 2h and its amount increased gradually with reaction time. During the last stages of reaction, -C2SH, as reported by41 and calcite also appeared. In Figure 1B the XRD patterns of metakaolinite and the resulting as-synthesized products after hydrothermal reaction with CaO solutions are illustrated. Metakaolinite showed a poor crystalline nature, with low-intensity peaks corresponding to quartz and muscovite, and was characterized by the appearance of an amorphous aluminosilicate (see the broad hump at 2θ=13-33°). According to Ríos et al.42 this persists between 600 and 950°C, with relicts of the original kaolinite and mullite as the main crystalline phase at 1000°C. After metakaolinite/lime reaction, the most important hydrated phases were Ca(OH)2, hydrogarnet, C2ASH8 and C4AH13 at the early stages. Metastable phases like C2ASH8 and C4AH13 were also reported by Frías et al.24 C-S-H, -C2SH, hydrogarnet and tobermorite were observed at the late stages. For comparison, a third mixture of pure chemical reagents (precipitated SiO2, Al2O3 and CaO) was prepared and its as-synthesis products are illustrated in Figure 2. Ca(OH)2, C-S-H, katoite and calcite were the phases identified at 0 h, along with relicts of kaolinite. Reinik et al.43 reported also the occurrence of katoite after hydrothermal alkaline treatment of oil shale ash. With curing time, katoite disappeared and new hydrated phases formed, which included C-S-H, hydrogarnet and tobermorite. It is evident that in the three investigated systems, hydrogarnet invariably appeared before 11Å tobermorite as reported in previous studies. Although the occurrence of metastable phases like C2ASH8 and C4AH13 is still unclear, we suggest that they were quickly converted to hydrogarnet, followed by tobermorite, with curing time. Le Saoût44 reported the occurrence of the polymorphs of CaCO3 (calcite an aragonite), with calcite as the main crystalline material at the surface. Our XRD analysis did not detected it (except by traces of calcite in Figure 2 due to its very small crystal size, although the thermogravimetric analysis reveals the presence of CaCO3 as will be discussed later. The occurrence of CaCO3 can be explained by dissolution of carbon dioxide in the solution producing CO32- ions, which react with Ca2+,44 and the Ca2+ ions required by these reactions are obtained by the dissolution of C-H and by lowering the Ca/Si ratio of the C-S-H.45
Several poorly crystalline materials, with un-reacted Ca(OH)2 occurred at shorter reaction times. Hydrogarnet was always among the first phases formed and invariably appeared before 11Å tobermorite. However, later, with increasing reaction time, they started to fracture and their quantity reduced almost in half over 24h. With increasing reaction time, hydrogarnet tended to undergo breakdown and its amount decreased and finally disappeared; CaO present in the further reaction with SiO2 formed hydrated calcium silicates, and released Al3+ ions were inserted into the Al-substituted tobermorite crystal lattice.27 Different research groups have classified calcium silicate hydrates based on morphological and chemical data to compensate for scarce structural data. Taylor46 classified C-S-H as C-S-H(I) and C-S-H(II) based on the Ca: Si ratio, with C-S-H(I) being obtained with Ca: Si 1.5 and C-S-H(II) produced using Ca: Si 1.5. Therefore, we consider that C-S-H(II) is the metastable phase that coexists with hydrogarnet and tobermorite, taking into account that we have used Ca: Si 1.5 to prepare gel compositions, as precursors of the synthetic calcium silicate hydrate phases obtained in the present study.
Scanning electron microscopy
SEM microphotographs of as-synthesized products autoclaved at 175°C for 0 and 24h. Synthesis products obtained from kaolinite/lime mixtures are characterized by the occurrence of flaky crystals of relict kaolinite associated with C-S-H and progressively with reaction time the breakdown of hydrogarnet, which in general shows an apparently intact surface. The typical octahedral morphology of hydrogarnet occurring in twinned crystals, developing clusters, obtained after metakaolinite/lime mixtures, whereas with reaction time tobermorite, showing typical Hebel microstructure, grew onto the hydrogarnet (rounded morphology) surface prior to its breakdown.
Fourier transformed infrared spectroscopy
The structure of C–S–H gel is very complex. FT-IR has been found to be very useful in delineating the complex chemistry involved in the cement due to the poor crystallinity of silicate hydrates.47 The FT-IR spectra in the region of 1200-400cm–1 of the as-synthesized products from the investigated mixtures for kaolinite and metakaolinite for a mixture of precipitated SiO2 Al2O3, using CaO as activator. The broad bands centred at 1115–1117cm–1 suggest the presence of C–S–H gels. This band decreased in intensity and exhibited a slight shift to lower frequencies with reaction time, and disappeared after 1.5h. Al–O stretching vibrations of tetrahedral AlO4 groups lie at 1020–990cm–1. The band at 970cm–1 is attributed to Si–O stretching vibrations in C–S–H gels with jennite type structure.48 The shoulder at 914-918cm–1 may be assigned to OH bending vibrations in Al–OH–Al bonds (octahedral aluminium). The band appearing at 798-800cm–1, which is assigned to stretching vibrations modes of O–T–O groups (T=Al, Si), is attributed to polymerization and Si replacement by Al in tetrahedral sites of C–S–H. The bands of at 873-879 and 696-700cm–1 can be attributed to calcite.
Solid-state nuclear magnetic resonance
Hydration phases like calcium hydroxide (C-H) and calcium-silicate-hydrate (C-S-H) are closely linked, although C-H and C-S-H are considered as crystalline and nearly amorphous materials, respectively. Therefore, its structure must be studied by other methods and/or by analogy with natural or synthetic minerals such as zeolites. Some information on the structure of these compounds can be obtained by MAS-NMR. 29Si and 27Al NMR were performed on CaO-activated raw materials, revealing important information on the structure of the as-synthesized hydrated phases.
The 29Si and 27Al NMR spectra of kaolinite and its synthesis products. The 29Si NMR spectrum of kaolinite a single resonance centred at -91.4ppm, which is characteristic of layer silicates and assigned to Si linked via oxygens to three other Si atoms.49–50 The chemical shift values of the 29Si NMR spectra of the treated kaolinite for 0 and 24h are indicating the occurrence of silicon sites of calcium silicate hydrate phases. A single resonance line at -91.3ppm, which can be attributed to the silicon site Q3(1Al) of Ca(OH)2, whereas three resonance lines at -79.5, -85.3 and -91.0ppm, which according to previous studies51–52 can be assigned to the silicon sites Q1, Q2(1Al) and Q3(1Al) of C–S–H phases, respectively. The 27Al NMR spectrum of kaolinite consists of a single resonance at -3.4ppm that is assigned to 6-coordinated Al. 27Al NMR spectra of the treated kaolinite, with a single resonance at -3.7ppm attributed to 6-coordinated Al and three resonance lines at 2.0, 11.6 and 55.4ppm; the double peak (composed by the first two resonances) is assigned to 6-coordinated Al, while the last one is attributed to 4-coordinated Al.
The 29Si and 27Al NMR spectra of metakaolinite and its synthesis products. The 29Si NMR spectrum of metakaolinite displays a broad resonance in the chemical shift range from -75 to -125ppm, centred at -96.3ppm, which can be assigned to the variety of silicon sites with Si linked to four other Si atoms in silica polymorphs53 and indicates the presence of amorphous silica.41 According to Mackenzie et al.54 when kaolinite is dehydroxylated, the Si atoms undergo a range of environments of different distortion and the broadness of the metakaolinite line is attributed to these variations in the Si-O-Si(Al) bond angles. The chemical shift values of the 29Si NMR spectra of the treated metakaolinite for 0 and 24h respectively. A single resonance line at -91.2ppm, being attributed to the silicon site Q3(1Al) of Ca(OH)2, whereas two resonance lines at -79.4 and -85.6ppm attributed to the silicon sites Q1 and Q2(1Al) of C-S-H phases. The 27Al NMR spectrum of metakaolinite displays two other resonances at -0.3ppm (assigned to 6-coordinated Al) and 23.4ppm (attributed to 4-coordinated Al, which are, however, different compared with those reported by Rocha et al.49 & Jiugao et al.50 The broader and asymmetrical peak shapes of metakaolinite show its disordered structure.55 27Al NMR spectra of the treated metakaolinite show a single resonance at -1.9ppm attributed to 6-coordinated Al and three resonance lines at 3.9, 11.7 and 55.6ppm; the double peak (composed by the first two resonances) is assigned to 6-coordinated Al, while the last one is attributed to 4-coordinated Al.
29Si NMR data reveal that silicon of the raw materials is completely converted with reaction time into C-S-H phases, including hydrogarnet and tobermorite. 11Å tobermorite exhibits two resonances at -85.7 and -95.7ppm attributed to chain middle group (Q2) and branching site (Q3).51 We consider that the resonances at -85.3 and -85.6ppm can be assigned to 11Å tobermorite. However, the lack of the resonance at -95.7ppm reported by Wieker et al.51 can be explained by an overlapping of resonances corresponding to different calcium silicate hydrates. Komarneri et al.52 showed that the substitution of aluminium in tobermorite caused a low field shift of the Q3 units from -95.7 to -91.5ppm, which can also explain the occurrence of the resonance at -91.0ppm. According to, whereas 27Al NMR data indicate the appearance of tobermorite at longer reaction time (24h), although it is difficult to suggest the occurrence of Al-substituted tobermorite or other Al-substituted C-S-H phase, as has been reported by other studies.52–56
Thermogravimetric analysis (TGA)
Thermogravimetry (TG) and derivative thermogravimetry (DTG) have proven to be valuable tools for evaluating the nature of hydration products according to different stages of cement hydration, as well as quantifying the different phases.57–61 The identity of the amount of the hydrated phases was estimated from thermogravimetric analysis. Hydration products, mainly calcium silicate hydrates (C-S-H gel) and portlandite (Ca(OH)2), are the result of hydration of the main components of cement. The hydration rate of cement can be evaluated by measuring the mass loss of hydrated compounds 700°C. Different DTG peaks or temperature ranges were obtained when the C-S-H gel was heated between 25 and 700°C: 100°C, dehydration of pore water; 100-250°C, different stages of C-S-H dehydration; 250-350°C, presence of a member of the hydrogarnet group; 350-500°C, decomposition of Ca(OH)2; 500°C, dehydroxylation of Ca(OH)2; 573°C: crystalline inversion of quartz; 700°C, decarbonation of CaCO3. A large amount of water is released between 100 and 250°C when the C-S-H gel began to decompose. According to Klimesch et al.22 hydrogarnet amount increased slightly up to 2h, followed by a decrease clearly demonstrating that this phase was decomposed and consumed with reaction time. C-S-H formation was enhanced with the use of the metakaolinite/lime mixture as indicated by the size and presence of several endotherms, compared with the other two mixtures, which indicates that the SiO2 source originating from metakaolinite is more reactivity than that from precipitated SiO2 and kaolinite.
Tobermorite appeared after 2h of autoclaving and increased with reaction time. However, the addition of metakaolinite to the mixture promoted a decrease in tobermorite formation. A weight loss of 23.36, 27.06 and 22.70% was obtained when kaolinite, metakaolinite and precipitated SiO2 Al2O3, respectively, were activated with CaO. The metakaolinite/lime mixture produced the highest weight loss, which indicates that this mixture had high water contents. However, it should be noted that the weight loss is overlapped by calcium silicate hydrates, which are known to dehydrate gradually and differently over a wide temperature range 800°C.62 Differences in their dehydration behaviour are due to compositional and structural variations.63
In general, the results of the thermogravimetric analysis indicates that the addition of CaO to kaolinite and metakaolinite produced several water containing phases in the temperature range 25-350°C. On the other hand, the DTA curves show the decomposition of portlandite (at 440°C) and calcite (at 680°C). Two interesting aspects observed are: a crystalline inversion of quartz (peak at 551°C and the presence of C2ASH8 and C4AH13 as reported by Frías et al.24
AMD treatment using kaolinite-based calcium silicate hydrates
The surface drainage waters at Parys Mountain are strongly acidic (pH 2) and metal-rich due to the oxidation of sulphide minerals, and its orange-brown colour is due to the very high concentrations of ferric iron in solution. Neutralization is generally the first step in treating AMD. The neutralization reaction of AMD was examined measuring the pH and electrical conductivity of sorbent: AMD mixtures (0.25g/20ml) over a period of 30 minute. pH and electrical conductivity trends obtained after treatment of AMD. KAOAds-3 produced the highest pH (10.87), whereas using KAOAds-7 as an adsorbent, the lowest pH (4.55) was obtained, which indicates that the sample KAOAds-3 showed the best neutralization capacity, due to the presence of portlandite as the main calcium silicate hydrate phase. However, pH increased as a consequence of the progressive dissolution of the sorbent (kaolinite-based calcium silicate hydrates) during the shaking process. The electrical conductivity of the treated AMDs did not show strong fluctuations or changes compared with raw AMD.
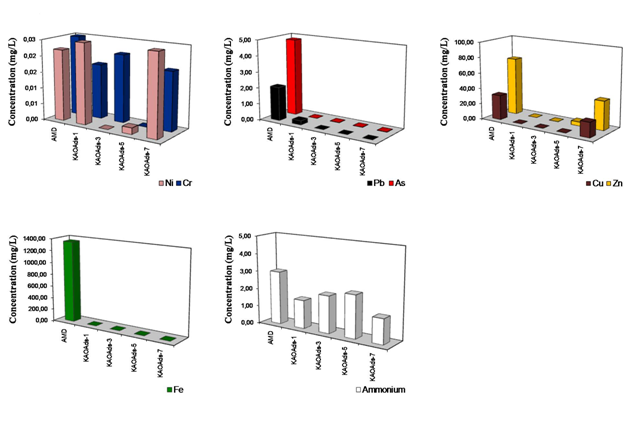
The removal of metal ions (Cu, Pb, Zn, Ni, Cr, As and Fe) and ammonium from AMD using kaolinite-based calcium silicate hydrates for a sorbent: AMD mixture of 0.25g/20ml. The removal trends of metal ions in the final leachates for the investigated mixtures are described as follows. Cu was almost completely removed using all samples, except KAOAds-7, which produced the higher Cu concentration (19.17ppm), whereas KAOAds-1 (portlandite) produced the lower residual concentration of Cu (0.029ppm). Pb shows a reversal behaviour compared with that observed for Cu, with a progressive decrease of concentration with curing time, which means that KAOAds-1, containing a single phase (portlandite) had a lower efficiency in Pb removal compared with KAOAds-7, which is composed of several calcium silicate hydrate phases like hydrogarnet and tobermorite. Zn displayed similar behaviour to that observed for Cu, although the highest residual concentration (37.230ppm) was obtained using KAOAds-7. Ni, Cr and as did not show consistent trends, which is evident from the slight fluctuations. The addition of the mixtures KAOAds-3 and KAOAds-5, which are characterized by the occurrence of several calcium silicate hydrates, except tobermorite, produce higher concentrations of Fe compared with those obtained using portlandite (KAOAds-1) and KAOAds-7. The final ammonium concentration obtained for the same mixtures. The samples KAOAds-1 and KAOAds-7 produced the lower residual concentration values, particularly the second one that contains tobermorite, whereas the higher concentration of ammonium was obtained after addition of KAOAds-5, which contains various calcium silicate hydrates (except tobermorite). Preliminary results demonstrate that the kaolinite-based calcium silicate hydrates can be used for remediation of AMD by removing heavy metals and ammonium.
Different C-S-H materials were synthesized in the system CaO-SiO2-Al2O3-H2O under hydrothermal conditions at 175°C for a monitoring time of 24h. The morphological, chemical and structural data determined by several analytical techniques are consistent and show good agreement with the literature. Some of the advantages to use an Al2O3 source such as kaolinite or metakaolinite is that Al accelerates tobermorite formation from C-S-H, inhibits the conversion to xonotlite, which reduces the strength properties of the synthetic product, and extends the temperature range over which tobermorite is stable.64 Metakaolinite is a more reactive phase than kaolinite for release of Al to form Al-tobermorite.
Several poorly crystalline materials occurred with un-reacted Ca(OH)2 at shorter reaction times. Hydrogarnet and tobermorite are interesting phases formed in the treated samples at longer curing times, which should promote the strength development of the synthesis products. Hydrogarnet was always among the first phases formed and invariably appeared before 11Å tobermorite. However, later, with increasing reaction time, they start to fracture and their quantity reduced almost in half during 24h.
Our results indicate that hydrogarnet is always one of the first phases formed. However, its continued existence in the final product depends on factors, such as reaction time and bulk composition. In the subsequent autoclaving process the reaction continues and tobermorite is formed, which is known to provide hardness and strength to the final material.
The thermal stability of the as-synthesized pastes shows the process of dehydration of free water and decomposition of the C-S-H gel from 25 to 250°C. The weight losses between 400 and 500°C are due to the decomposition of portlandite. The decomposition of carbonate phases occurred at 500°C.
It is well known that calcium silicate hydrates exhibit a good capability to fix metals and metalloids. Tobermorite is a calcium silicate hydrate substituted with Al3+ and alkali, which exhibits cation exchange and selectivity properties for Cs and Rb, falling between those of clay minerals and zeolites. Unlike clay minerals and zeolites, tobermorite is expected to be thermodynamically stable in cement and concrete which have a similar chemical composition and therefore, it will be suitable for inexpensive solidification in cement after their use in the decontamination of radioactive species from nuclear wastes. Therefore the capabilities of those phases to retain heavy metals should be evaluated. A batch sorption study confirmed that the C-S-H-bearing products were effective in the exclusion of metal ions and ammonium from AMD. The as-synthesized C-S-Hs could be recognized as materials with potential applications in heavy metal removal from polluted effluents. However, in spite of their cation exchange capacity represents a very important characteristic quality in the removal of undesired species from aqueous solutions, it is not the deciding factor affecting the performance this cation exchanger during ion exchange processes, since numerous other factors also need to be considered.
The first author would like to thank the Programme AlBan, the European Union Programme of High Level Scholarships for Latin America (scholarship no. E05D060429CO), and the Universidad Industrial de Santander (remunerated commission). This research has benefited from research facilities provided by the School of Applied Sciences at the University of Wolverhampton.
The author declares no conflict of interest.

©2017 Ríos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.